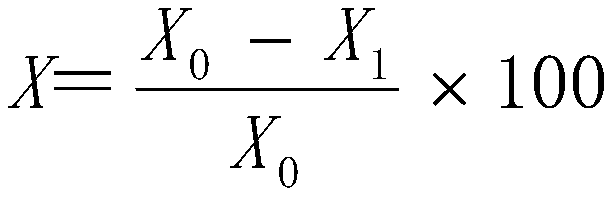Patents
Literature
37 results about "Ethylenediamine tetra-acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Environment-friendly rustproof cleaning agent
The invention provides an environment-friendly rustproof cleaning agent prepared from the following raw materials in percentage by weight: 2-5% of one or mixture of multiple of phosphoric acid or phosphate, 2-5% of one or mixture of multiple of boric acid or borate, 0-5% of strong base, 0.5-1% of anionic surface active agent, 0.5-1% of nonionic surface active agent, 0.5-1% of one or more than one of edetic acid or ethylenediamine tetraacetates and the balance of water. According to the invention, the raw materials are added into water in sequence to obtain the rustproof cleaning agent. The environment-friendly rustproof cleaning agent provided by the invention is safe and environment-friendly and has good dustproof cleaning capability.
Owner:东莞市赛亚气雾剂有限公司
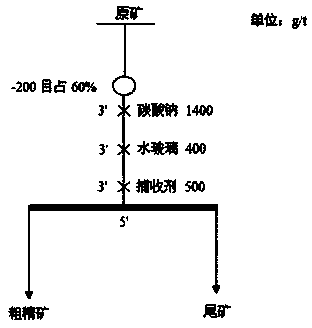
Mineral flotation collecting agent for oxidized ores
The invention provides a collecting agent for collecting minerals of oxidized ores such as ilmenite and a preparation method thereof, and belongs to the technical field of mineral flotation collectingagents. The mineral flotation collecting agent for the oxidized ores such as ilmenite is used after ammonium dibutyl dithiophosphate, ethylenediaminetetraacetic acid salt, maleate, sodium oleate andthe like are mixed; and the mineral flotation collecting agent has the characteristics of good water solubility, good dispersion and high selective adsorption ability to objective minerals, and is mainly used for flotation separation of valuable minerals in the oxidized ores such as the ilmenite, scheelite and wolframite. The preparation method of the mineral flotation collecting agent for the oxidized ores is simple in process, operation is easy to control, the prepared collecting agent has the advantages of high selectivity, good dispersion, low agent dosage and high mineral separation comprehensive efficiency, and quite important significance for promoting mineral separation of the minerals such as the oxidized ores is achieved.
Owner:河南天鸿新材料科技有限公司
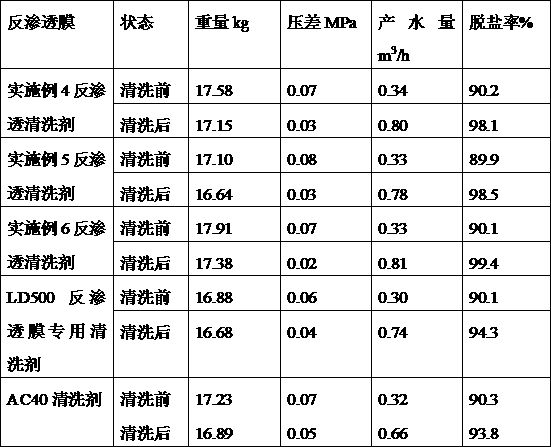
Reverse osmosis cleaning agent for seawater desalination equipment as well as preparation method and use method thereof
ActiveCN113134305AEfficient removalHas a chelating effectGeneral water supply conservationSeawater treatmentOrganic baseCitrate salt
The invention discloses a reverse osmosis cleaning agent for seawater desalination equipment, a preparation method and a use method, and belongs to the technical field of seawater desalination. The reverse osmosis cleaning agent comprises an acidic agent A and a multifunctional agent B; wherein the acidic agent A is composed of citric acid, hydrochloric acid, formic acid, nitrilotriacetic acid trisodium salt and deionized water, and the multifunctional agent B is composed of inorganic base, ethylenediamine tetraacetic acid salt, polyethyleneimine, alcohol amine organic base and the like; According to the invention, the acidic agent A and the multifunctional agent B are cooperatively used for cleaning the reverse osmosis membrane, during use, the multifunctional agent B is firstly used for removing various organic impurities, micro-plastics and acidic substances in the reverse osmosis membrane, then the multifunctional agent B in the reverse osmosis membrane is washed away through water washing, and then the acidic agent A is used for removing various metal salts, metal ions and metal oxides. Finally, thereverse osmosis membrane is washed with a multifunctional agent B to fully remove a small amount of organic impurities, and meanwhile, a certain repairing effect on the reverse osmosis membrane is achieved.
Owner:山东九莹环境工程有限公司
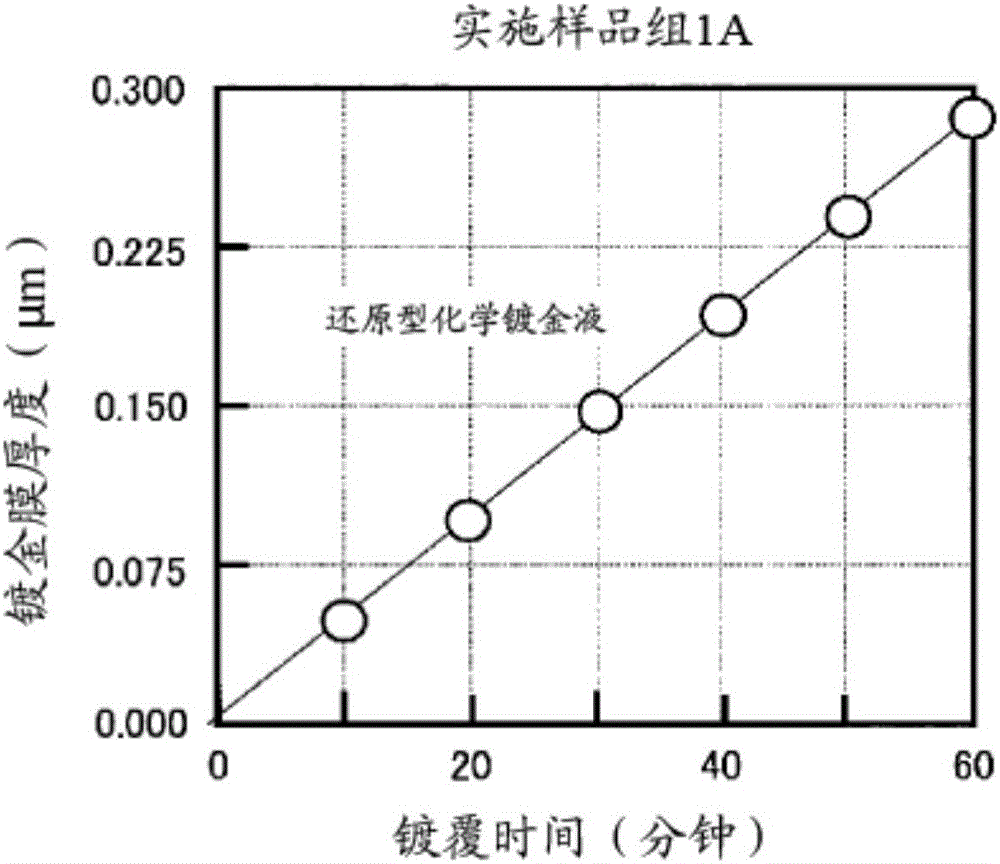
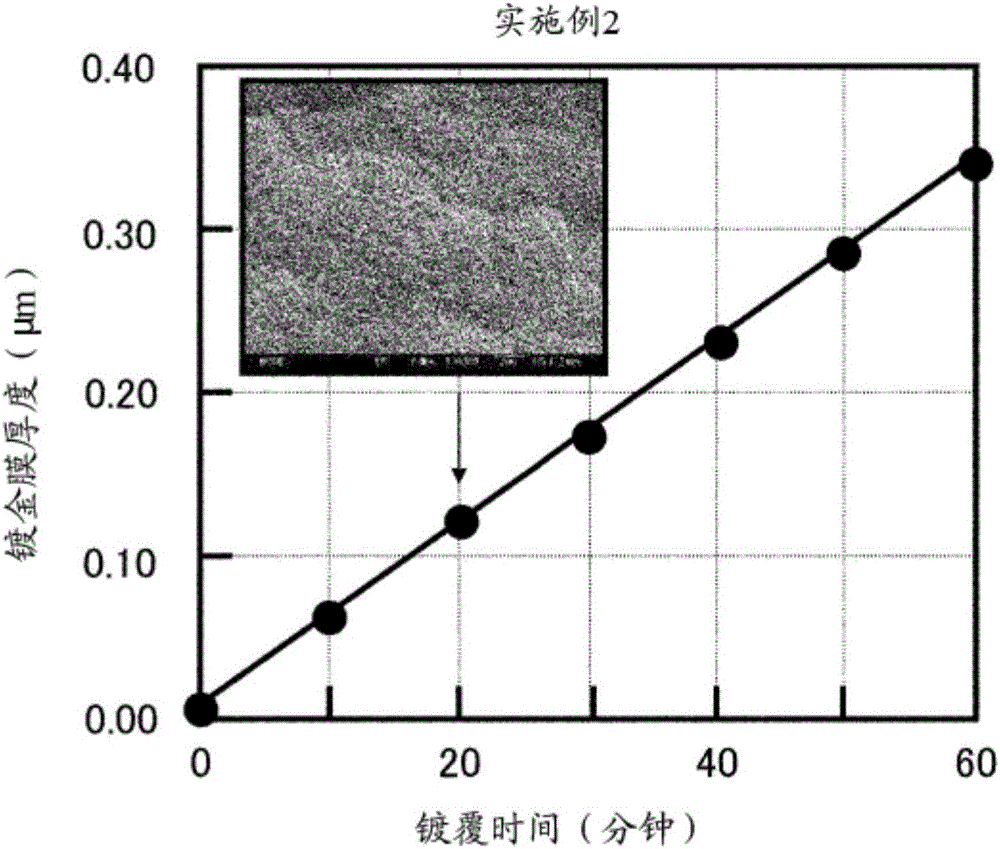
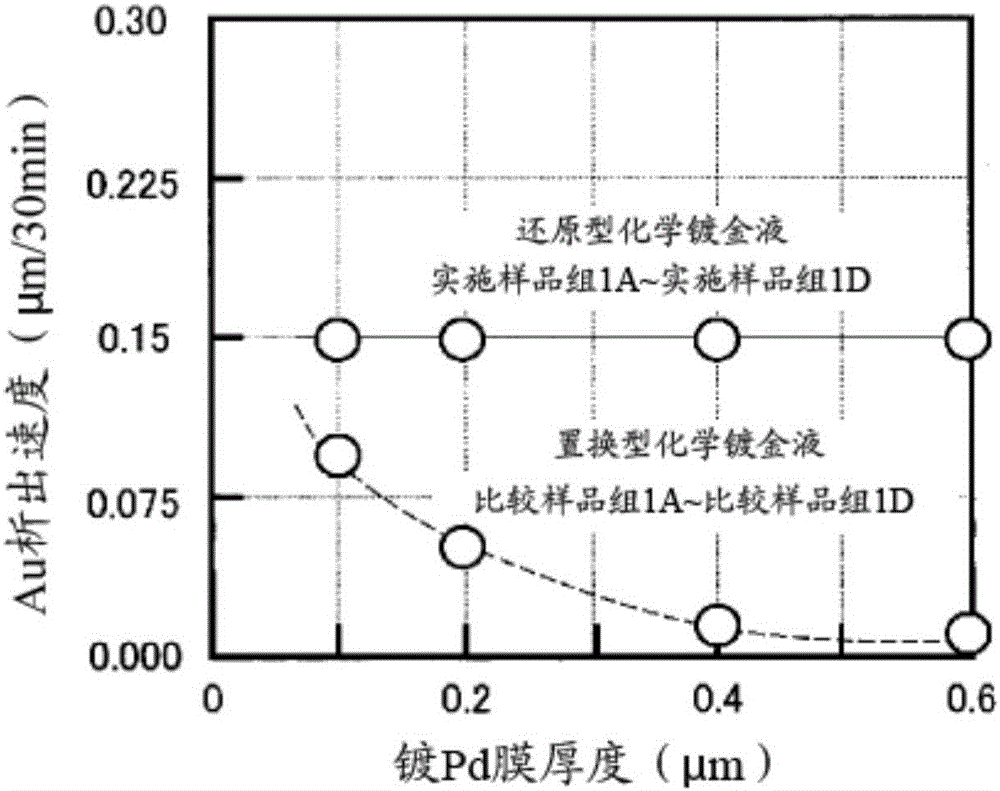
Reduction-type electroless gold plating solution and electroless gold plating method using said plating solution
ActiveCN105745355AGood adhesionImprove joint reliabilityLiquid/solution decomposition chemical coatingHexamethylenetetramineWire bonding
The purpose of the present invention is to provide an electroless gold plating solution which does not contain harmful substances and is capable of achieving good wire bonding performance, while suppressing corrosion of a base metal. In order to achieve this purpose, an electroless plating solution that contains a water-soluble gold compound, citric acid or a citrate, ethylenediaminetetraacetic acid or an ethylenediaminetetraacetate, hexamethylenetetramine and a chain polyamine containing three or more amino groups and an alkyl group having three or more carbon atoms is employed as a reduction-type electroless gold plating solution which is used for the purpose of forming an electroless gold plating film on the surface of an object to be plated by means of electroless plating.
Owner:KOJIMA CHEM CO LTD
Preparation method of prussian white analogue with low defects and low water content
The invention discloses a preparation method of a prussian white analogue with low defects and low water content, the chemical formula of the prussian white analogue is KxMn[Fe(CN)6]1-y.zH2O, the value range of x is 1.7-2.0, the value range of y is 0-0.05, and the value range of z is 0.05-0.3. The preparation method comprises the following steps: dissolving potassium ferrocyanide in water to obtain a solution A; dissolving a soluble divalent manganese salt and a complexing agent ethylenediamine tetraacetic acid salt in water to obtain a solution B; and adding the solution B into the solution Aunder the conditions of reaction atmosphere and stirring the mixed solution at room temperature, and carrying out standing, filtering, washing and drying treatment to obtain the low-defect and low-water-content Prussian white analogue. According to the invention, the Prussian white analogue with low defect and low water content is prepared by adopting a complexing-assisted synthesis method, so that the thermal stability of the material and the electrochemical cycling stability of the material in a potassium ion battery are improved.
Owner:BEIHANG UNIV
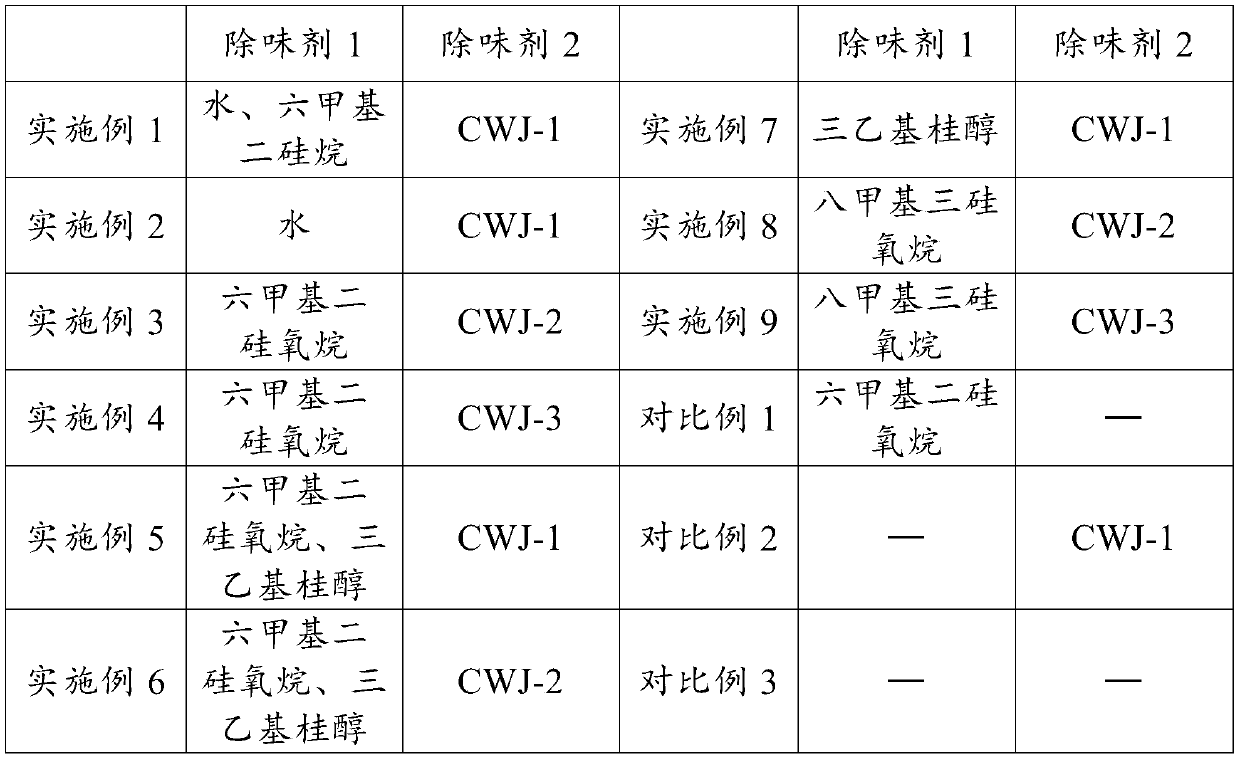
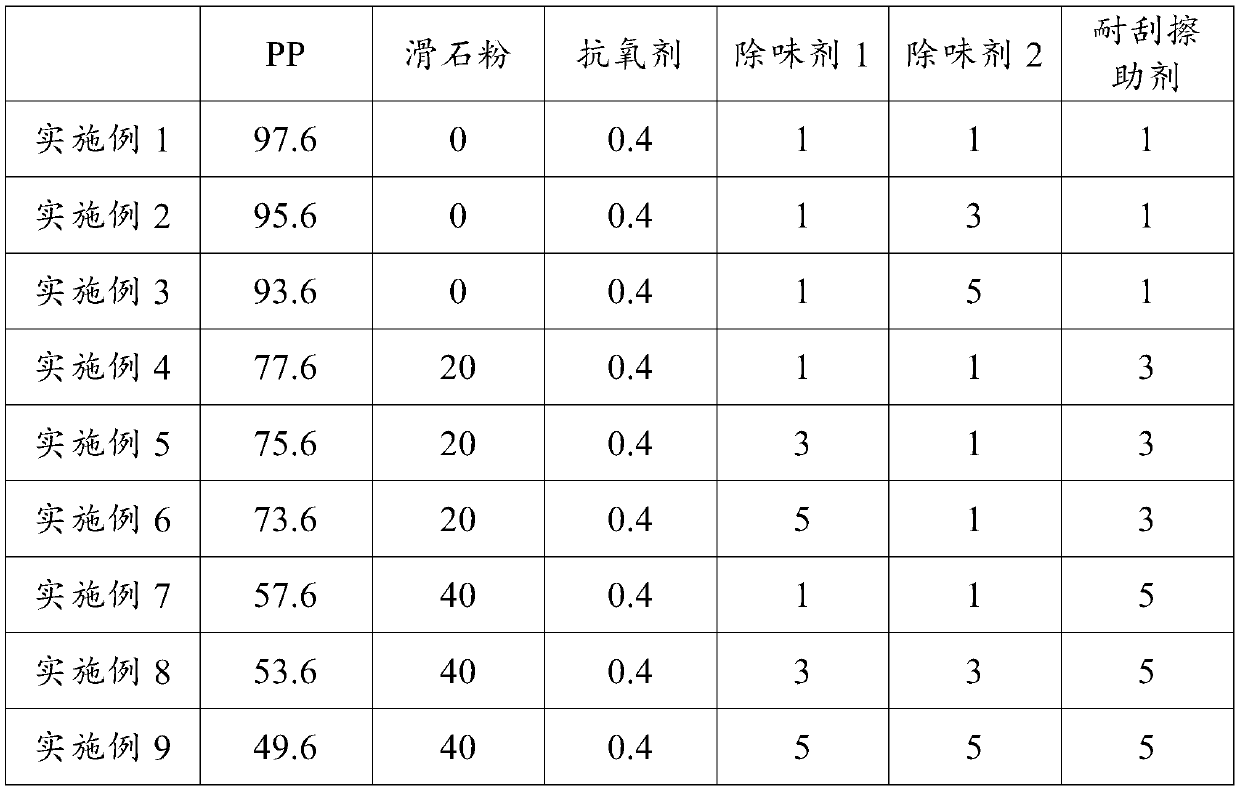
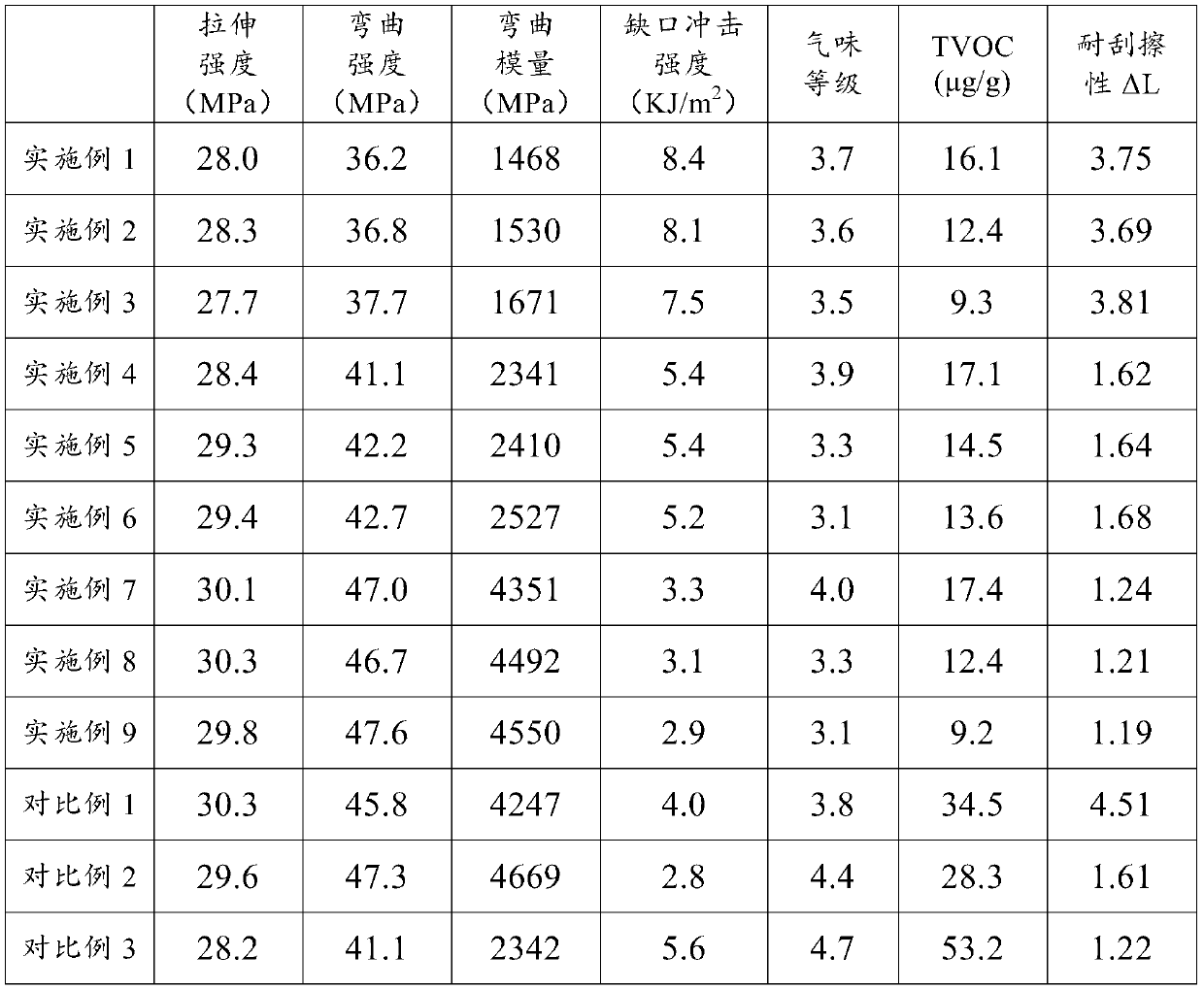
Low-odor scratch-resistant polypropylene composite material and preparation method thereof
The invention relates to a low-odor scratch-resistant polypropylene composite material, which is prepared from the following raw materials by mass: 44.6-96.6% of polypropylene, 0-40% of talcum powder,0.4-4% of an antioxidant, 1-5% of a deodorant 1, 1-5% of a deodorant 2 and 1-5% of a scratch-resistant aid, wherein the deodorant 1 is one or a plurality of materials selected from water, hexamethyldisilane, hexamethyldisiloxane, triethylsilanol and octamethyltrisiloxane, and the deodorant 2 is mesoporous silica or a 13X type zeolite molecular sieve loading at least one selected from ethylenediamine tetraacetic acid salt, methylene triamine pentamethylene phosphoric acid, ethylenediamine tetramethylene phosphoric acid and 2,4-dinitrophenylhydrazine. According to the invention, the product hasexcellent low-odor and excellent scratch resistance, reduces the antagonism among odor, diffusivity and scratch resistance, and finally realizes the balance of odor, diffusivity and scratch resistance of the product.
Owner:中广核俊尔(上海)新材料有限公司
Cyanide-free silver-based composite plating solution, silver-based composite plating layer and preparation method thereof
ActiveCN111235608AImprove stabilityImprove anti-interference abilityElectrolytic coatingsCyanideActive agent
The invention discloses a cyanide-free silver-based composite plating solution, a silver-based composite plating layer and a preparation method of the silver-based composite plating layer. The cyanide-free silver-based composite plating solution comprises silver nitrate, a multicomponent complexing agent, conductive salt, a buffering agent, a cationic surfactant, an additive and micro-nano particles; the multielement complexing agent takes triethylenetetramine or / and soluble salt thereof as a main complexing agent, and takes one or more of triethylenetetramine hexaacetic acid or triethylenetetramine hexaacetic acid salt, ethylenediaminetetraacetic acid or ethylenediaminetetraacetic acid salt, and citric acid or citrate as an auxiliary complexing agent; the concentration of the silver nitrate is 0.1-0.7 mol / L, the concentration ratio of the main complexing agent to the silver nitrate is 1-6:1, the concentration of the auxiliary complexing agent is 0.05-1 mol / L, the concentration of theconductive salt is 0.2-1.2 mol / L, the concentration of the cationic surfactant is 0.0005-0.003 mol / L, and the buffering agent is used for the pH value of the cyanide-free silver-based composite plating solution to be 1-7. The cyanide-free silver-based composite plating solution is stable, and the prepared silver-based composite plating layer has the functions of friction reduction and wear resistance and has good compactness.
Owner:ELECTRIC POWER RES INST OF STATE GRID ZHEJIANG ELECTRIC POWER COMAPNY +2
Cyanide-free silver plating solution and silver plating layer and preparation method thereof
ActiveCN111235609AStrong complexationChange in polarization resistanceCellsActive agentSurface-active agents
The invention discloses a cyanide-free silver plating solution and a silver plating layer and a preparation method thereof. The cyanide-free silver plating solution provided by the invention comprisessilver nitrate, a multiple complexing agent, conductive salt, a buffering agent and an additive, wherein the multiple complexing agent takes triethylene tetramine or / and the soluble salt of the triethylene tetramine as a main complexing agent, takes one or more of triethylenetetramine hexaacetic acid or triethylenetetraamine hexaacetic acid salt, ethylene diamine tetraacetic acid or ethylenediaminetetraacetic acid salt, citric acid or citrate as an auxiliary complexing agent; the concentration of the silver nitrate is 0.1- 0.7mol / L, the concentration ratio of the main complexing agent to thesilver nitrate is 1- 6:1, the concentration of the auxiliary complexing agent is 0.05- 1mol / L, the concentration of conductive salt is 0.2- 1.2mol / L, and the bufferring agent is used for adjusting thepH value of the cyanide-free silver plating solution to 2- 7; the additive is a rare earth salt or a mixture of the rare earth salt and benzotriazole or / and surfactant, the concentration of the additive is 0.0001- 0.035mol / L. The cyanide-free silver plating solution provided by the invention has good stability, and the prepared cyanide-free silver plating layer has a dense structure.
Owner:ELECTRIC POWER RES INST OF STATE GRID ZHEJIANG ELECTRIC POWER COMAPNY +3
Alkaline cleaner special for reverse osmosis membrane and application method
The invention provides an alkaline cleaner special for a reverse osmosis membrane. The alkaline cleaner special for the reverse osmosis membrane comprises nitrilotriacetic acid salt, complexonate, hydroxyethyl ethylene diamine, (2-Hydroxyethyl) ethylenediaminetriacetic acid salt, alcamines, a dispersant, a pH conditioning agent and deionized water, wherein the mass ratio of nitrilotriacetic acid salt, complexonate, hydroxyethyl ethylene diamine, (2-Hydroxyethyl) ethylenediaminetriacetic acid salt, alcamines, the dispersant to the pH conditioning agent is 1-10 to 2-10 to 2-15 to 10-40 to 10-30 to 2-10 and the balance is deionized water. The invention further provides an application method for the alkalinity cleaning agent special for the reverse osmosis membrane. The alkaline cleaner is convenient to use and excellent in effect, and causes no harm to membrane components.
Owner:WEIHAI XIANGYU TECH
Compound injection of Xylazine, and preparation technique
InactiveCN1957918AIdeal anesthesia brake effectEasy to usePharmaceutical delivery mechanismAnaestheticsAcetic acidDihydroetorphine hydrochloride
An injection of compound xylazine for the anesthesia of wild animal is prepared through dissolving xylazine and EDTA in distilled water, adding the solution of dihydroetorphine hydrochloride, stirring, filling it in ampules, and sterilizing.
Owner:闫章年
Composite quality control product for gastric function detection and detection kit
InactiveCN111024964AMeet stability requirementsShelf life stableDisease diagnosisBiological testingPepsinogen IPepsinogen II
The invention relates to a pepsinogen I, pepsinogen II and gastrin-17 composite quality control product. The composite quality control material comprises a protein preserving solution, and pepsinogenI, pepsinogen II and gastrin-17 which are dissolved in the protein preserving solution, wherein the total volume of the protein preserving solution is calculated; wherein the protein preserving solution contains 0.8 to 3.5 g / L of AEP-HBC, 0.002 to 0.006 g / L of fibrinogen, 0.05 to 0.15 g / L of gelatin, 2 to 8 g / L of trehalose, 0.05 to 0.25 g / L of tetrapolyphosphate, 5-25 mmol / L of 2-(N-morpholine) ethanesulfonic acid, 0.5-4 g / L of ethylenediaminetetraacetic acid or ethylenediaminetetraacetic acid salt, 1-20 mmol / L of cysteine, methionine or arginine and 0.05-0.10% of a preservative,. The pepsinogen I, pepsinogen II and gastrin-17 composite quality control product is initiated at home, and can be stably stored for 14 months at the temperature of 2-8 DEG C. The invention further discloses a preparation method of the pepsinogen I, pepsinogen II and gastrin-17 composite quality control product. The invention also provides a detection kit containing the composite quality control product, andthe detection kit can be used for clinical detection of gastric functions.
Owner:深圳市蔚景生物科技有限公司
Citric acid discoloration incrustation cleaning agent and preparation method and application thereof
InactiveCN110982644AReduce concentrationImproves the ability to remove scaleOrganic detergent compounding agentsSurface-active detergent compositionsOrganic acidCooling tower
The invention relates to a citric acid discoloration incrustation cleaning agent and a preparation method and application thereof. The cleaning agent is prepared from the following components in massconcentration: 15%-25% of organic acid, 1.6%-2.0% of ammonium salt, 0.03%-0.05% of ethylenediamine tetraacetic acid salt, 0.003%-0.005% of a surfactant, 0.4%-0.7% of a corrosion inhibitor and 0.00003%-0.00005% of an indicator. The cleaning agent is applied to removing incrustation of boilers, cooling towers or circulating water pipelines, and when effective components of the cleaning agent are consumed, the cleaning agent can be turned into purple from orange to indicate that the cleaning agent fails. Compared with the cleaning agents in the prior art, the cleaning agent has the advantages oflow corrosion to equipment, high cleaning capacity, safety, no pollution, low maintenance cost and the like.
Owner:SHANGHAI INST OF TECH
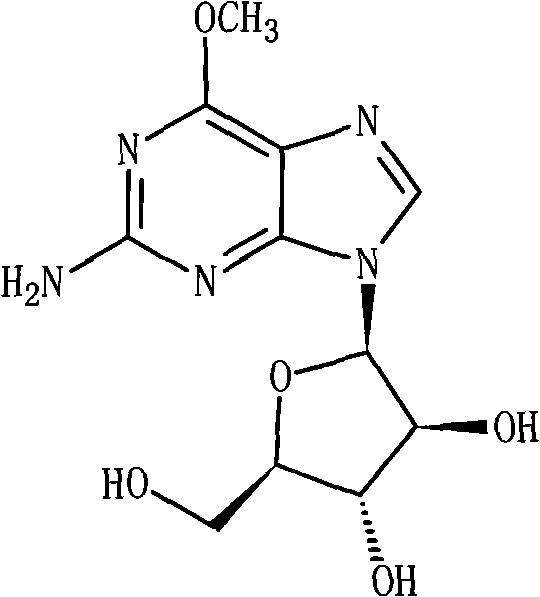
Nelarabine injection
InactiveCN101401786AImprove stabilityLow content of related substancesOrganic active ingredientsPharmaceutical delivery mechanismBottleInjection solution
The invention relates to a nelarabine injection, which is characterized by consisting of nelarabine, EDTA or EDTA salt, sodium chloride, and water for injection, wherein the nelarabine accounts for 0.25 to 0.625 percent of the total weight of the injection, the EDTA or the EDTA salt accounts for 0.001 to 0.01 percent of the total weight of the injection, the content of the sodium chloride is 0 or the dose is used for regulating the osmotic pressure, and the balance is the water for injection. The preparing process comprises the following steps: taking 80 percent of the water for injection, adding raw supplementary materials into the water for injection, stirring the mixture to fully dissolve the raw supplementary materials and mixing the mixture evenly, adjusting the pH value, adding 0.02 percent of needle activated carbon into the mixture, keeping the water temperature at 70 DEG C, stirring the mixture for 30 minutes, filtering the mixture to remove the activated carbon when the mixture is hot, cooling the mixture to room temperature, measuring the contents and the pH value of the solution, adding the water for injection with full dose into the solution, mixing the mixture evenly, filtering the mixture through 0.22 mu m of microfiltration membrane until the mixture is clear, packaging the filtrate into infusion bottles after intermediates are measured to be qualified, plugging the bottles, and rolling the mouths of the bottoms to obtain the nelarabine injection after hot pressing sterilization.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Graphene modified concrete retarder and preparation method thereof
The invention belongs to the technical field of concrete admixtures, and relates to graphene modified concrete retarder and a preparation method thereof. The retarder disclosed by the invention is prepared from the following components in parts by weight: 10 to 40 parts of graphene, 5 to 35 parts of ethylenediamine tetraacetic acid salt, 10 to 30 parts of formate, 10 to 35 parts of calcium bromideand 10 to 20 parts of urea. The retarder has the characteristics that the concrete setting time is delayed, the concrete strength increasing speed is increased, and the strength of concrete at all ages is improved.
Owner:HOHAI UNIV
Stabilized efinaconazole compositions
The present invention provides compositions containing efinaconazole, butylated hydroxytoluene, a salt of ethylenediaminetetraacetic acid, and optional citric acid. The compositions exhibit stable color profiles and are useful in the treatment of fungal infections.
Owner:BAUSCH HEALTH IRELAND LTD
Metal surface cleaning agent and preparation method thereof
The invention discloses a metal surface cleaning agent and a preparation method thereof. The cleaning agent is prepared by mixing alkylphenol polyvinyl ether, a defoaming agent, alcohol ether, hydrogen peroxide, ethylene diamine tetraacetic acid salt, pearl powder, sodium hydroxide and deionized water. The preparation method comprises the following steps: 1, weighing alkylphenol polyvinyl ether, the defoaming agent, alcohol ether, hydrogen peroxide, ethylene diamine tetraacetic acid salt, pearl powder, sodium hydroxide and deionized water; 2, mixing the alkylphenol polyvinyl ether, alcohol ether, ethylene diamine tetraacetic acid salt, sodium hydroxide and deionized water, stirring and heating for 2-6 minutes, and naturally cooling to obtain a mixed liquid I; 3, adding the pearl powder into the mixed liquid I, and stirring for 12-16 minutes to obtain a mixed liquid II; and 4, adding hydrogen peroxide into the mixed liquid II, stirring, and finally adding the defoaming agent to obtain the cleaning agent. The cleaning agent has strong oil stain removing capability and little foam, and can be normally used at low temperature.
Owner:WUXI EPIC TECH
Chelating fluid for enhanced oilrecovery in carbonate reservoirs and method of using the same
The chelating fluid for enhanced oil recovery in carbonate reservoirs and the method of using the same utilizes a chelating fluid injected into a carbonate oil reservoir through a fluid injection system. The chelating fluid is a solution of a polyamino carboxylic acid chelating agent in brine, with the polyamino carboxylic acid chelating agent having a concentration of approximately 5.0 wt % of the solution, with the solution having a pH of approximately 11.0. The polyamino carboxylic acid chelating agent is preferably ethylenediaminetetraacetic acid (EDTA). The brine preferably has a relatively high salinity, with a sodium chloride concentration preferably on the order of approximately 18,300 ppm.
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS
Emulsion medicine injection of propofol and its prepn process
ActiveCN1846690AGeneration of controlObvious combination synergyHydroxy compound active ingredientsAnaestheticsDrugChemistry
The present invention relates to emulsion medicine injection of propofol comprising propofol, metal ion chelating agent and cysteine. The present invention solves well the problem of the oxidation of phenol hydroxyl group in effective component propofol to produce degradation product and can ensure safety of clinical administration.
Owner:SICHUAN KELUN PHARMA CO LTD
A flooding system suitable for high temperature and high salinity reservoirs
The invention discloses an oil displacement system suitable for high-temperature and high-salt oil reservoirs. The oil displacement system is prepared according to the following method: in an inert atmosphere, in sodium formate, disodium edetate, ferrous sulfate, tetramethylethylenediamine, 4,4'-azobis (4-cyano Under the condition that base valeric acid), ammonium persulfate and hydrogen peroxide exist, monomer A, monomer B, monomer C, monomer D and inorganic component carry out copolymerization reaction in water, obtain gel; Said gel After chopping, add sodium silicate and sodium pyrophosphate, let stand to swell, dry and pulverize the swollen gel to obtain powder, which is the oil displacement system. The monomers adopted in the invention (all of which are acryloyloxy monomers) have relatively high activity, are easy to prepare high-molecular-weight products, have excellent viscosity-increasing properties, and are used in small amounts. The oil displacement system of the invention is easy to store and transport, and meets the requirements of environmental protection. The raw materials of the method of the invention are easy to obtain, and the preparation process is suitable for industrial production.
Owner:CHINA NATIONAL OFFSHORE OIL (CHINA) CO LTD +1
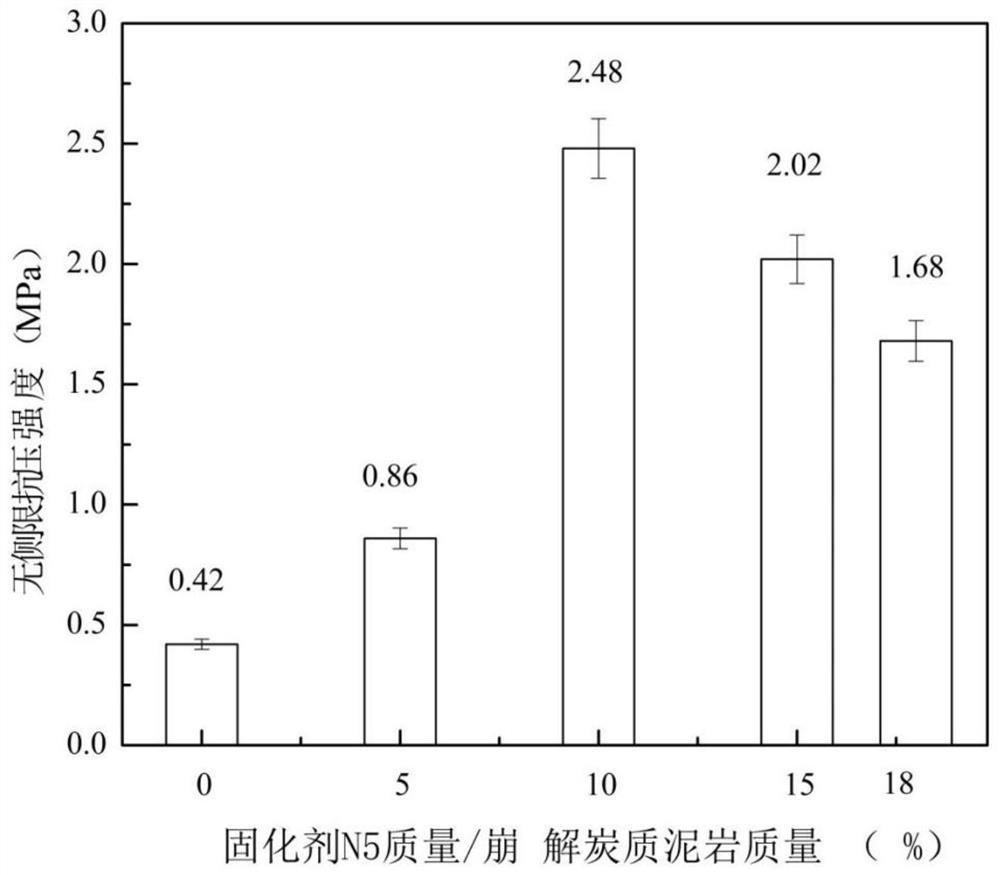
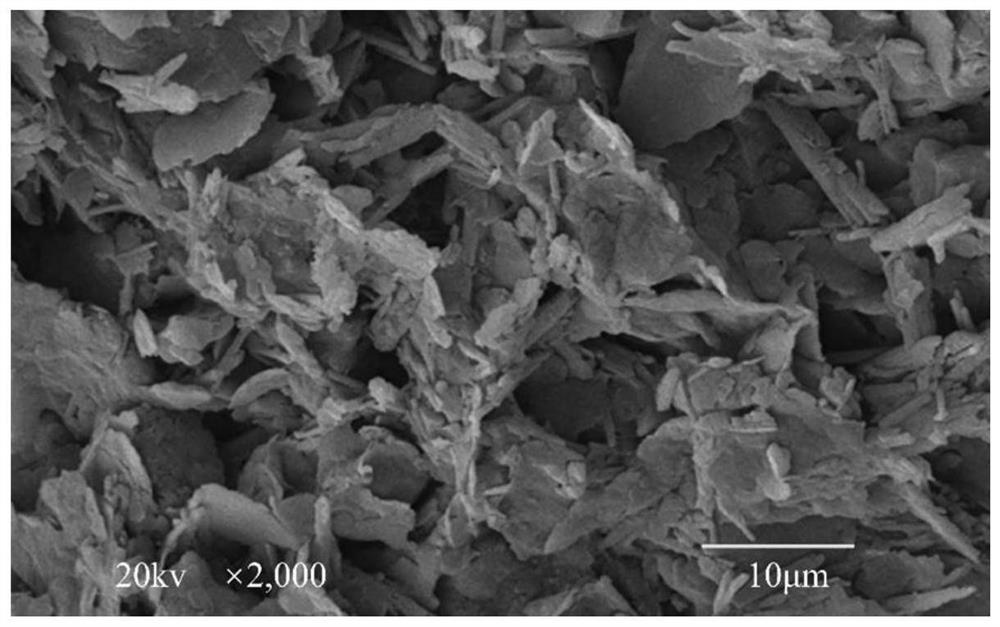
Nano-fiber curing agent for disintegrating carbonaceous mudstone and its preparation and use methods
The invention discloses a nano-fiber curing agent for disintegrating carbonaceous mudstone and its preparation and use methods. The nano-fiber curing agent for disintegrating carbonaceous mudstone consists of the following parts by weight: 30-40 parts of cement , 2-5 parts of nanomaterials, 5-10 parts of organic gel, 0.5-1 parts of disodium edetate, 25-35 parts of slag, 2-6 parts of fiber materials, and 16-25 parts of water. The nano-fiber curing agent for disintegrating carbonaceous mudstone provided by the present invention is an inorganic-organic composite curing agent, and the cured disintegrating carbonaceous mudstone has unconfined compressive strength under the synergy of various components The advantages of high quality and good structural integrity realize the reuse of slag while reducing the amount of cement, low cost, energy saving and pollution reduction.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method of extracting body fluid of crab
InactiveCN102440206AHigh in proteinLess impuritiesClimate change adaptationPisciculture and aquariaOxalateAnticoagulant Agent
The invention discloses a method of extracting body fluid of a crab, which belongs to the field of biotechnology and includes puncturing a living body of the crab with a needle of a syringe to slightly extract body fluid of the living body of the crab, immediately putting the extract into a container in which ethylenediaminetetraacetic acid anticoagulant solution or oxalate anticoagulant solution is added preliminarily, adjusting addition amount of the anticoagulant solution to control concentration of the coagulant in the extract to be within a certain range, fully mixing the anticoagulant solution with the extract prior to filtering by means of a filter medium, centrifuging the mixture at a rotation speed of 4000rpm / min-8000rpm / min to obtain clear liquor, finally adding phosphate buffer with a pH range from 5.5 to7.5 into the clear liquor to obtain the stored body fluid of the crab, and temporarily reserving the stored body fluid of the crab in a refrigerating chamber of 0 DEG C to 4 DEG C or reserving the stored body fluid of the crab for a long time at -80 DEG C to -20 DEG C. The method is simple in operation and free of expensive equipment, and the body fluid of the crab is high in protein content and has fewer impurities.
Owner:GUANGDONG OCEAN UNIVERSITY
A kind of decontamination moisturizing baby wet tissue and preparation method thereof
ActiveCN110974717BHas antibacterial cleaning effectQuick removalCosmetic preparationsToilet preparationsGlycerolWet wipe
Owner:WALCH GUANGZHOU COMMODITY
Ketamine-containing pharmaceutical composition as well as preparation method and application thereof
ActiveCN113813250APromote absorptionWell mixedOrganic active ingredientsNervous disorderCelluloseEthylene diamine tetra acetic
The invention relates to a ketamine-containing pharmaceutical composition as well as a preparation method and application thereof. The ketamine-containing pharmaceutical composition comprises the following components in parts by weight of 16000 to 16500 parts of ketamine; 120 to 180 parts of citric acid; 5 to 15 parts of ethylene diamine tetraacetic acid salt; 40 to 100 parts of an adsorbent; 2 to 8 parts of Tween 80; 0.1-4 parts of a preservative; and the absorbent is a cellulose adsorbent. The ketamine-containing pharmaceutical composition disclosed by the invention can realize a very good drug effect through nasal administration.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
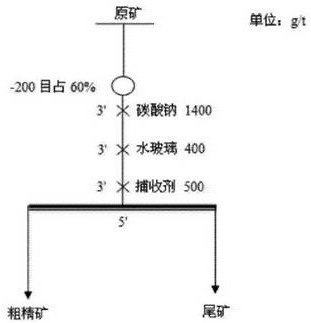
A kind of oxidized ore mineral flotation collector
ActiveCN109078762BIncrease ore loadHigh selectivityFlotationDibutyl dithiophosphateMineral flotation
The invention provides a collecting agent for collecting minerals of oxidized ores such as ilmenite and a preparation method thereof, and belongs to the technical field of mineral flotation collectingagents. The mineral flotation collecting agent for the oxidized ores such as ilmenite is used after ammonium dibutyl dithiophosphate, ethylenediaminetetraacetic acid salt, maleate, sodium oleate andthe like are mixed; and the mineral flotation collecting agent has the characteristics of good water solubility, good dispersion and high selective adsorption ability to objective minerals, and is mainly used for flotation separation of valuable minerals in the oxidized ores such as the ilmenite, scheelite and wolframite. The preparation method of the mineral flotation collecting agent for the oxidized ores is simple in process, operation is easy to control, the prepared collecting agent has the advantages of high selectivity, good dispersion, low agent dosage and high mineral separation comprehensive efficiency, and quite important significance for promoting mineral separation of the minerals such as the oxidized ores is achieved.
Owner:河南天鸿新材料科技有限公司
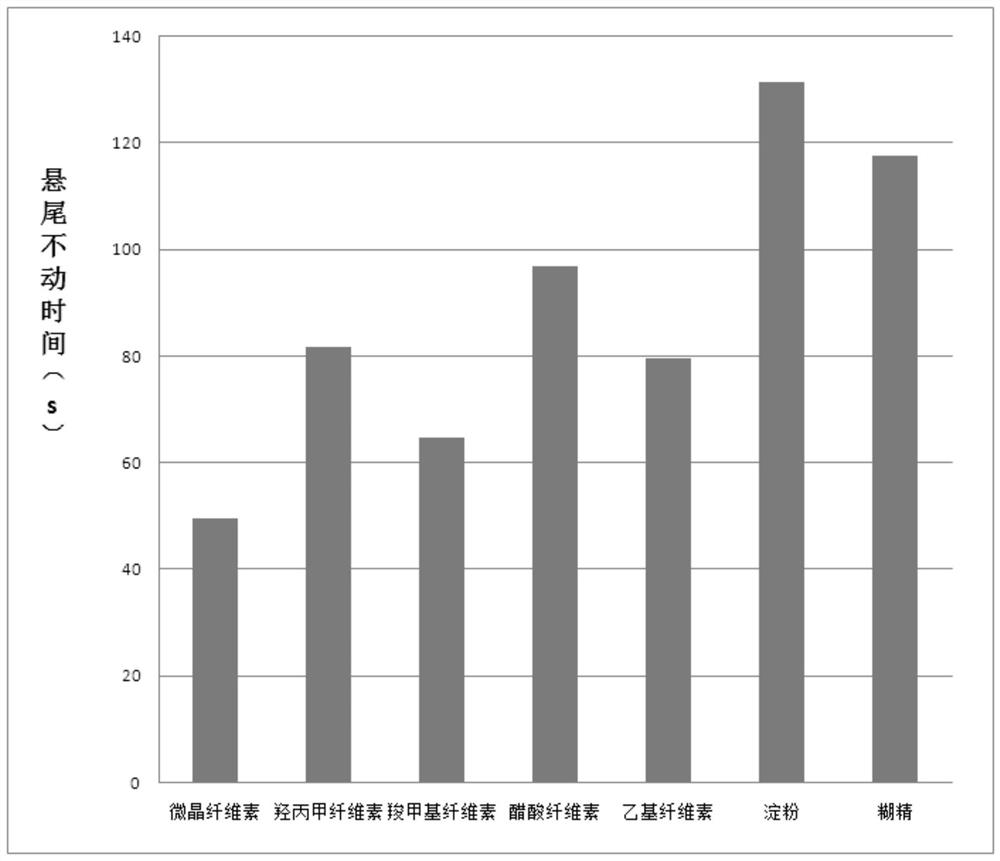
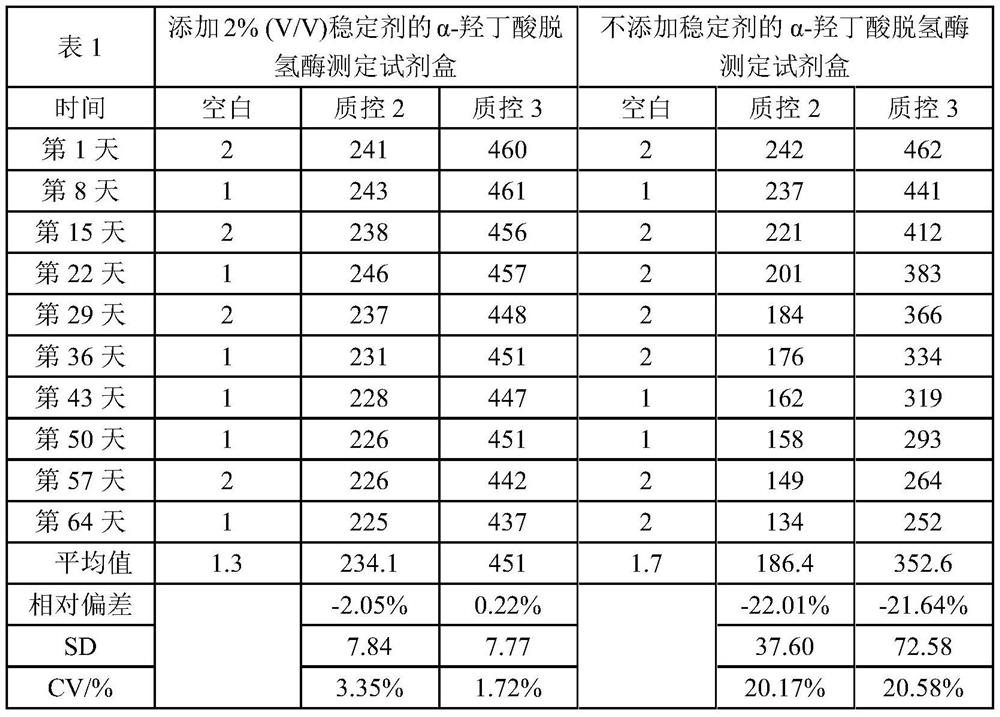
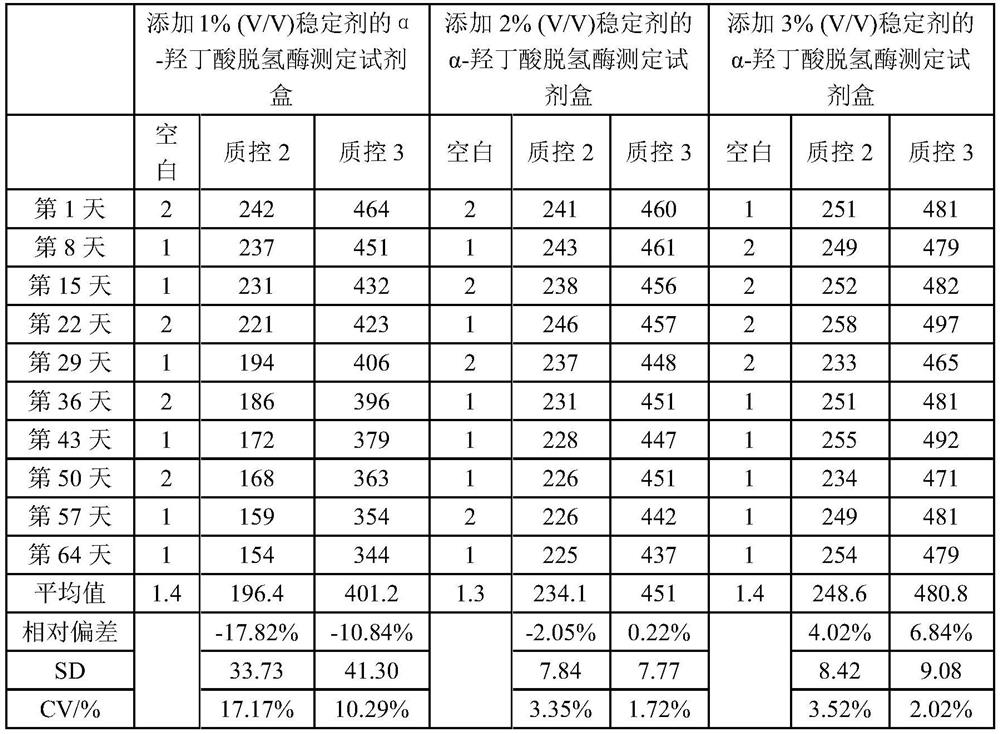
Stabilizer for α-hydroxybutyrate dehydrogenase assay kit and preparation method thereof
ActiveCN107991252BLong storage timeLow costChemical analysis using catalysisColor/spectral properties measurementsSucroseAssay
The present invention disclosed a stabilizer and preparation method for α -hydroxyl acid dehydrogenase testing kit, which belongs to the field of in vitro diagnostic.The stabilizer includes the following percentage components: 0.1 ~ 0.5 % ethyleine tetharolytic acid sodium, 1 to 3 % sucrose, 1 to 2 % Triton X‑100, 3 to 5 % CCD, 1 to 2 % glycerin, 0.1 ~ 0.2 % liquid biochemical PROCLIN300, the remaining amount of water.The stabilizer of the present invention is used in the α -hydroxyl acid dehydrogenase testing kit, which ensures the accuracy of the test results of the kit, and makes the alpha -hydroxyl acid dehydrogenase test box more stable, which can effectively extend the reagent reagentThe preservation time of the box.In addition, the cost of the stabilizer is low, the preparation is simple, and it is worth further promotion.
Owner:ZHONGSHAN CHUANGYI BIOCHEM ENG
Compound injection of Xylazine and preparation technique
InactiveCN100512817CEnsure anesthesiaGuaranteed normal brakingPharmaceutical delivery mechanismAnaestheticsFeral animalPharmacology
The invention provides a compound xiarazine injection and its preparation process. Put 100g of xylazine and 61-66g of ethylenediaminetetraacetic acid into double-distilled water, dissolve them at a temperature of 60-90°C, and prepare them according to the ratio of 5000-2500:1. Take dihydroetorphine hydrochloride and mix the solution evenly to make a mixed solution containing 100 mg of xylazine, 61-66 mg of ethylenediaminetetraacetic acid, and 10-20 μg of dihydroetorphine hydrochloride per ml, and fill it in an ampoule. Sterilized prepared. The invention provides a powerful braking drug for anesthesia and braking of wild animals, which can be used in industrial production and promote artificial breeding and wild animal protection.
Owner:闫章年
A kind of cyanide-free silver-based composite plating solution and silver-based composite coating and preparation method thereof
ActiveCN111235608BImprove stabilityImprove anti-interference abilityElectrolytic coatingsCyanideActive agent
The invention discloses a cyanide-free silver-based composite plating solution, a silver-based composite plating layer and a preparation method of the silver-based composite plating layer. The cyanide-free silver-based composite plating solution comprises silver nitrate, a multicomponent complexing agent, conductive salt, a buffering agent, a cationic surfactant, an additive and micro-nano particles; the multielement complexing agent takes triethylenetetramine or / and soluble salt thereof as a main complexing agent, and takes one or more of triethylenetetramine hexaacetic acid or triethylenetetramine hexaacetic acid salt, ethylenediaminetetraacetic acid or ethylenediaminetetraacetic acid salt, and citric acid or citrate as an auxiliary complexing agent; the concentration of the silver nitrate is 0.1-0.7 mol / L, the concentration ratio of the main complexing agent to the silver nitrate is 1-6:1, the concentration of the auxiliary complexing agent is 0.05-1 mol / L, the concentration of theconductive salt is 0.2-1.2 mol / L, the concentration of the cationic surfactant is 0.0005-0.003 mol / L, and the buffering agent is used for the pH value of the cyanide-free silver-based composite plating solution to be 1-7. The cyanide-free silver-based composite plating solution is stable, and the prepared silver-based composite plating layer has the functions of friction reduction and wear resistance and has good compactness.
Owner:ELECTRIC POWER RES INST OF STATE GRID ZHEJIANG ELECTRIC POWER COMAPNY +2
Preparation method of environment-friendly plant energy liquid
PendingCN113149749ADeliciousHigh in Vitamin CLiquid fertilisersFertilizer mixturesBacillus licheniformisInsect pest
The invention relates to a preparation method of environment-friendly plant energy liquid. The energy liquid is prepared from saccharomycetes, lactic acid bacteria, bacillus licheniformis, photosynthetic bacteria, ethylenediamine tetraacetic acid salt, inorganic salt, an additive, water and the like. The energy liquid is non-toxic and harmless, and can be directly sprayed on plants when in use. The energy liquid can significantly promote healthy growth of plants, increase the yield by 15-20%, significantly reduce plant diseases and insect pests, improve the quality of agricultural products and improve the taste. In addition, the energy liquid also can improve the soil, enhance the water retention capacity of the soil and solve the problems of soil acidification or hardening and the like. In addition, the preparation process of the energy liquid is simple, the whole preparation process is green and environment-friendly, and no three wastes are discharged.
Owner:山东天鹰生物科技有限公司
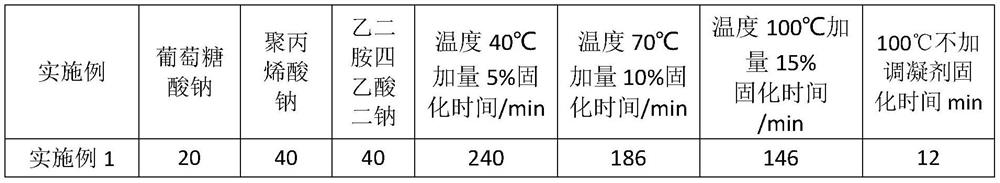
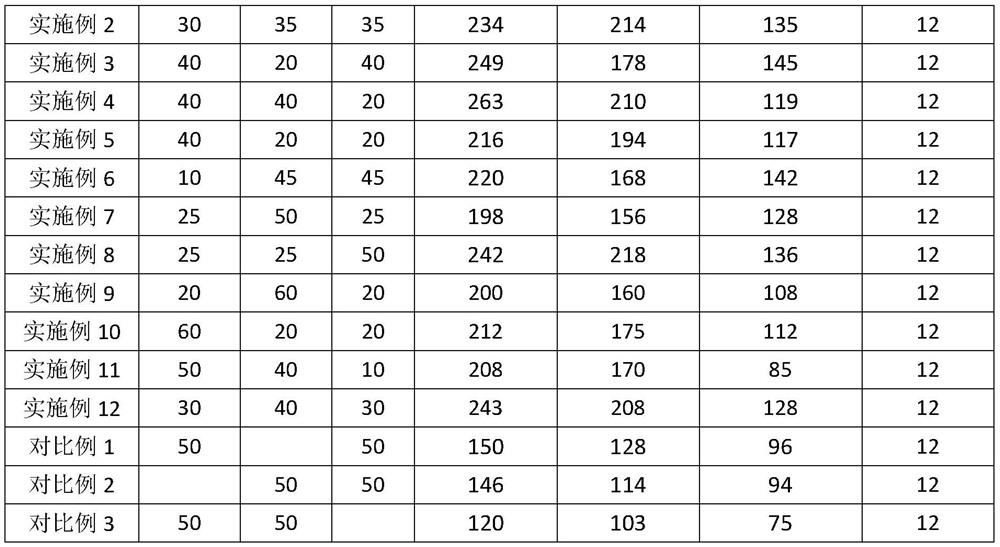
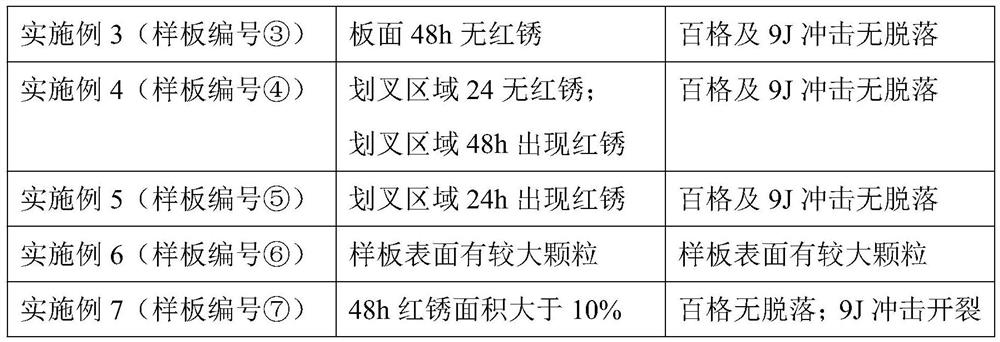
A kind of setting control composition, setting control agent and application thereof
ActiveCN112574729BImprove rheologyEasy cementing constructionDrilling compositionPolymer scienceWell cementing
The present invention relates to a coagulation control composition, coagulation control agent and application thereof. The coagulation adjusting composition provided by the present invention mainly comprises gluconate, polyacrylate and ethylenediaminetetraacetate. By applying the setting modifier provided by the invention to the thermosetting resin cementing material system, the thickening time of the thermosetting resin cementing material at a temperature of 30 to 100° C. can be adjusted to exceed 150 minutes, and the safety cementing of medium and shallow oil and gas wells within 4000 m can be satisfied. need.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of temporary antirust liquid for color-coated sheet
ActiveCN112724828AIncrease productivityAvoid exposureAnti-corrosive paintsLubricant compositionSilanesBenzioc acid
The invention relates to the field of color-coated sheets, and particularly discloses a preparation method of temporary antirust liquid for a color-coated sheet. The temporary antirust liquid is prepared from the following raw materials in parts by mass: 30-60 parts of water-based organic silicon emulsion, 0.5-5 part of cerium salt, 5-30 parts of silicate, 0.5-5 part of benzoate, 0.5-4 part of ethylene diamine tetraacetic acid salt and 30-70 parts of deionized water, wherein the water-based organic silicon emulsion is obtained by crosslinking three types of silane coupling agents containing epoxy groups, long-chain alkyl groups and amino groups. The temporary antirust liquid provided by the invention has corrosion resistance of a chemical conversion layer in a conventional process and excellent binding force with finish paint, and can be used for replacing two processes of the chemical conversion layer and a primer layer in the conventional process, so that the production efficiency of a color-coated sheet is improved while the cost is saved; and when the temporary antirust liquid acts on a cold-rolled steel sheet, a formed conversion film has certain self-repairing capability.
Owner:武汉迪赛新材料有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com