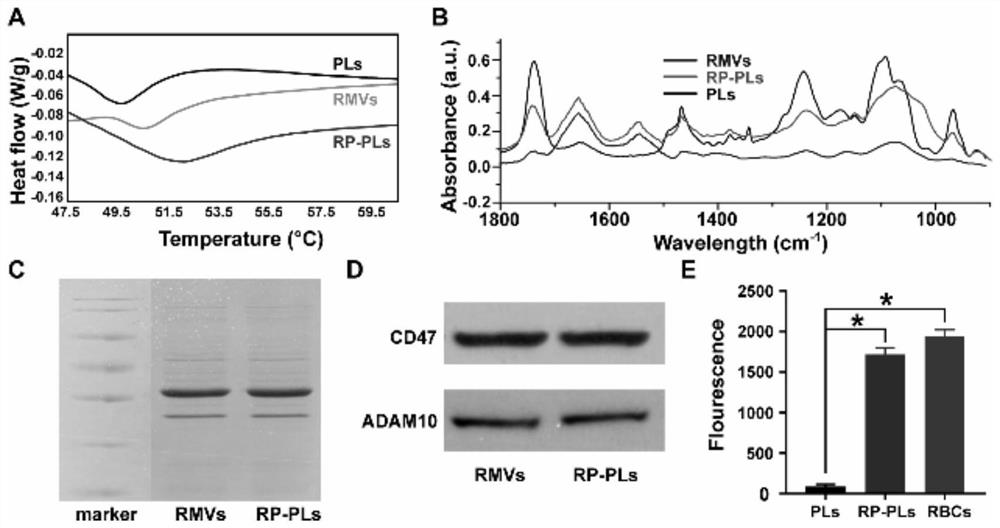
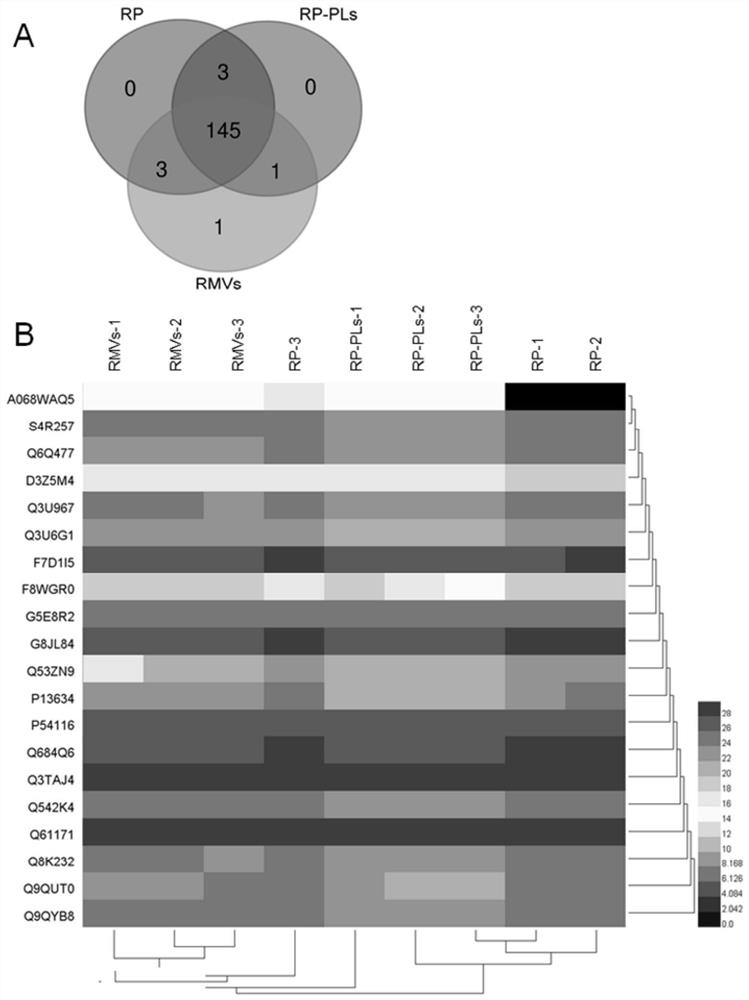
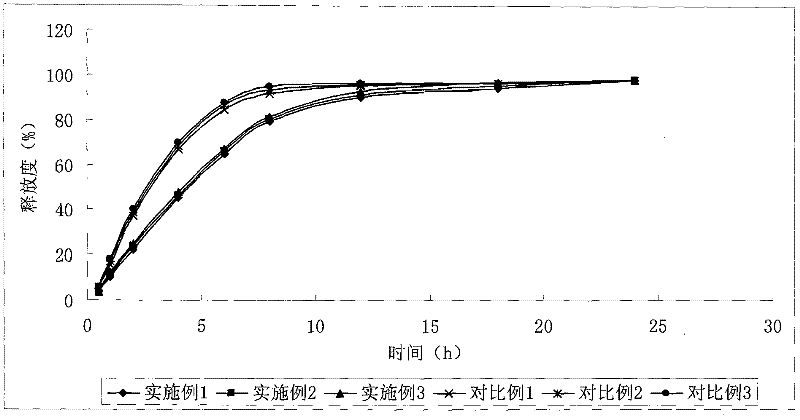
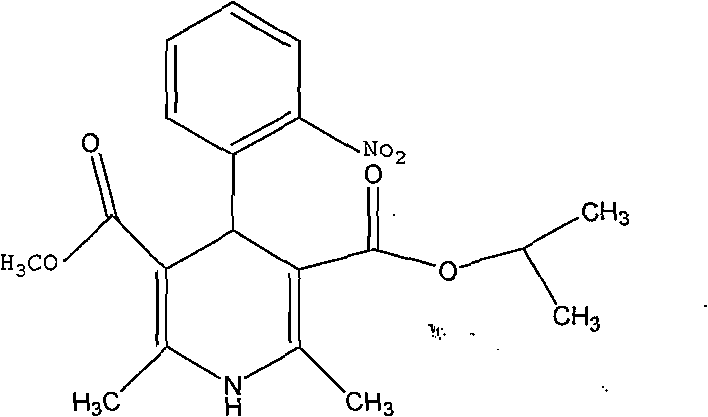
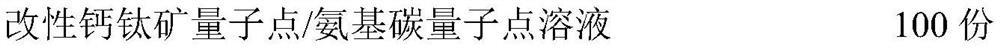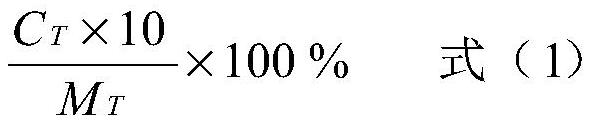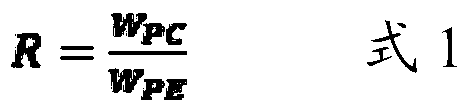Patents
Literature
80 results about "Phosphatidal ethanolamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
DSPE-PEG-FA-modified nanometer paclitaxel liposome and preparation method thereof
InactiveCN102188382AHigh encapsulation efficiencyGuaranteed purityOrganic active ingredientsPharmaceutical non-active ingredientsPaclitaxel InjectionDspe peg
The invention relates to a DSPE-PEG2000-FA-modified nanometer paclitaxel liposome. A molar ratio of the DSPE-PEG2000-FA to egg yolk lecithin is 0.05% to 0.15%, and a particle size of the liposome is less than 150 nm. A preparation method of the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome comprises the following steps: first, preparing a DSPE-PEG2000-FA into a DSPE-PEG2000-FA micelle; second, performing incubation on the phosphatidyl ethanolamine-polyethylene glycol2000-folic acid micelle and a paclitaxel liposome together to obtain a DSPE-PEG2000-FA-modified nanometer paclitaxel liposome. Materials, which are forbidden to be used in clinical practice, are not used as crude materials in the present invention. According to the invention, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome has a small particle size, and the content of the DSPE-PEG2000-FA is low; besides, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome has good drug entrapment efficiency and good colloid stability. Moreover, the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome can be absorbed effectively by an ovarian cancer cell having properties of sensitiveness to folic acid (+) and drug resistance, and the cytotoxicity of folic acid dependence is displayed; therefore, the efficacy of the DSPE-PEG2000-FA-modified nanometer paclitaxel liposome is stronger than that of a paclitaxel injection.
Owner:李红霞
Folate-targeted hydrophobic medicine-loaded polymeric vesicle and preparation method and use thereof
InactiveCN101953778AFlexibleGood flexibilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsFreeze-dryingPolyethylene glycol
The invention discloses a folate-targeted hydrophobic medicine-loaded polymeric vesicle and a preparation method and use thereof. The polymeric vesicle is prepared by the following steps of: (1) dissolving amphiphilic triblock copolymer and a hydrophobic medicine in an organic solvent, adding distearamide phosphatidyl ethanolamine-polyethylene glycol (2000) folate, evaporating to remove the organic solvent to prepare a uniform thin film, blowing and drying; and (2) adding double distilled water and products in the step (1) into a container, hydrating, shaking and uniformly mixing to ultrasonically form stable emulsion, performing film filtering, collecting filter liquor and performing freeze drying, The polymeric vesicle has main advantages of liposome, nanoparticles and other particle medicine-loaded systems, stability in vivo and in vitro obviously superior to the liposome, thicker film layer, contribution to encapsulating the hydrophobic medicine, dual-targeting function of EPR passive targeting and folate active targeting, and capacity of making the medicine-loaded vesicle effectively targeted and gathered on a tumor part and fulfilling the aim of targeted therapy.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
3-n-butylphthalide injection and preparation method thereof
ActiveCN103505409AEffectively mask special fragranceMask special fragranceOrganic active ingredientsPowder deliveryVinyl etherActive agent
The invention relates to a 3-n-butylphthalide injection and a preparation method thereof. The 3-n-butylphthalide injection disclosed by the invention comprises 3-n-butylphthalide or a derivant thereof, a surfactant and water for injection, wherein the surfactant is selected from one or more of phospholipid, polyethylene glycol-12-hydroxy stearate, polyethylene glycol-VE (Vinyl Ether) carbonic ester, polyethylene glycol-VE succinate, polyethylene glycol-DSPE (distearoyl phosphoethanolamine), polyethylene glycol-cholesteryl hemisuccinate, polyethylene glycol-cholesterol methyl ester, polyethylene glycol-cholesterol sulfate, polyoxyethylene dehydration sorbitol fatty acid ester and poloxamer. By adopting the 3-n-butylphthalide injection disclosed by the invention, a special fragrance of the 3-n-butylphthalide can be effectively concealed; the 3-n-butylphthalide injection is stable in quality, high in 3-n-butylphthalide content, simple in preparation technology, strong in operability and beneficial to industrialization, can be separately subpackaged into small volume of preparation, can be applied to intravenous injection, and also can be applied to intramuscular injection; the clinical drug delivery requirements are met.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
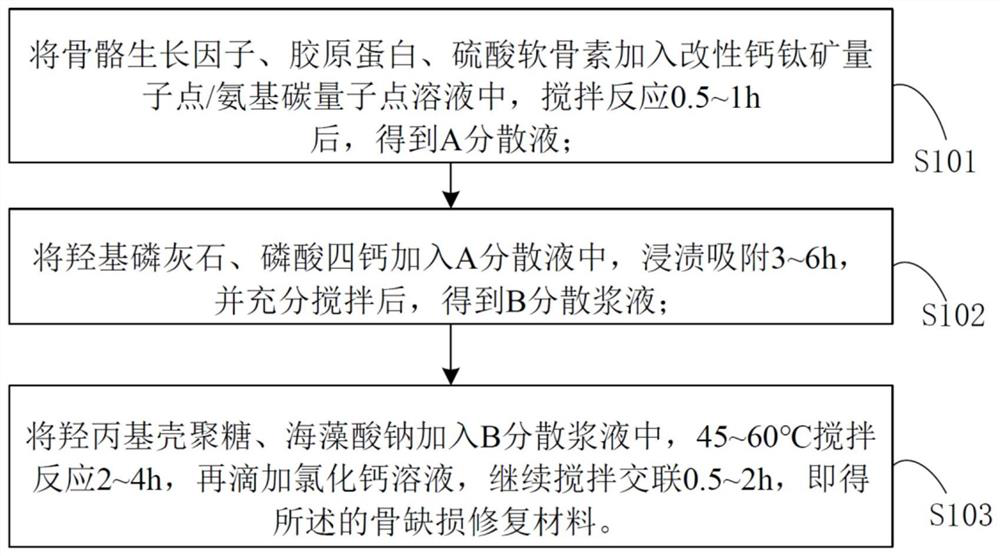
Bone defect repair material based on modified perovskite quantum dots/amino carbon quantum dots and preparation method thereof
InactiveCN112618788AStimulate proliferation and regenerationHigh fluorescence quantum efficiencyTissue regenerationProsthesisPhosphoric acidAcyl group
The invention discloses a bone defect repair material based on modified perovskite quantum dots and a preparation method of the bone defect repair material. The bone defect repair material comprises the following raw materials in percentage by mass: 20-30% of hydroxyapatite, 10-20% of octacalcium phosphate, 0.5-2% of bone morphogenetic protein, 1-5% of collagen, 0.5-2% of chondroitin sulfate, 3-5% of carboxymethyl chitosan and the balance of a modified perovskite quantum dot solution. The modified perovskite quantum dot / amino carbon quantum dot solution is prepared from the following reaction raw materials in parts by mass: 0.5 to 1.5 parts of perovskite quantum dots, 8 to 16 parts of dipalmitoyl phosphatidyl ethanolamine, 3 to 7 parts of acidic amino acid, 1 to 3 parts of reduced glutathione, 0.5 to 1 part of EDC.HCl, 20 to 30 parts of chloroform and 100 parts of deionized water. Perovskite quantum dots and amino carbon quantum dots are introduced into the bone defect repair material for the first time, stem cell transfection is mediated by quantum dot and stem cell behavior changes are monitored by utilizing a fluorescence microscopy imaging technology; meanwhile, the stem cells are induced to differentiate towards osteoblasts and chondroblasts, and proliferation and regeneration of the osteoblasts are stimulated.
Owner:蚌埠泰鑫材料技术有限公司
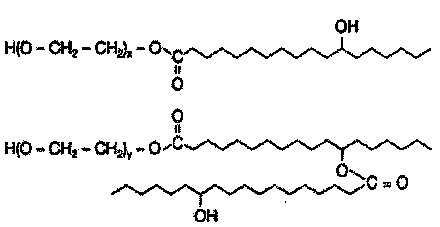
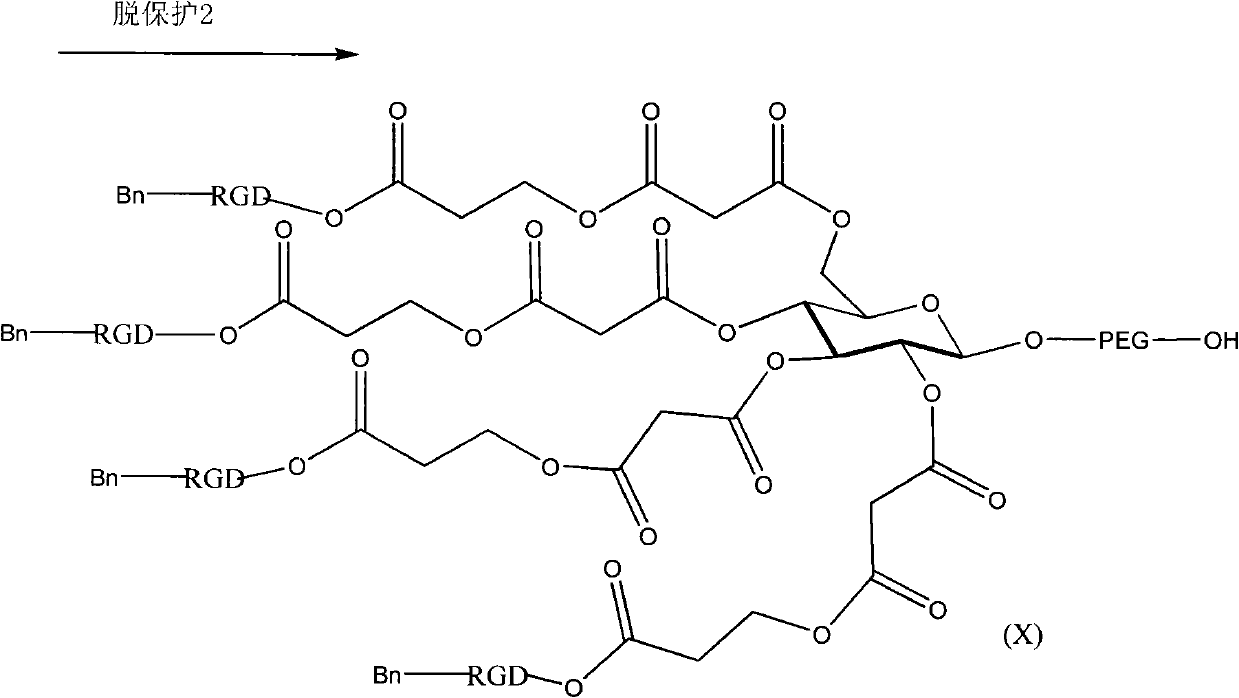
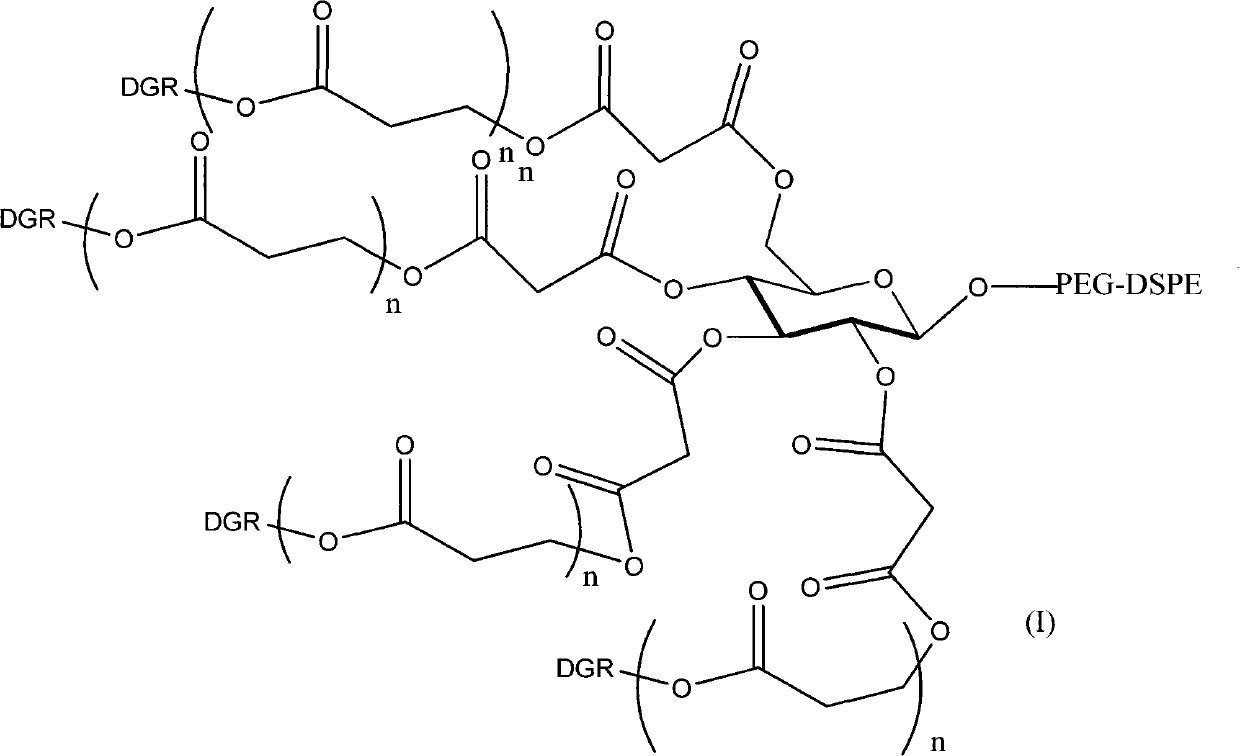
Polyethylene glycol-distearoyl phosphatidyl ethanolamine derivant and preparation method thereof
ActiveCN101864071AIncreased tumor affinityAdapt to treatment needsPharmaceutical non-active ingredientsPolyethylene glycolEthanolamines
The invention discloses a polyethylene glycol (PEG)- distearoyl phosphatidyl ethanolamine (DSPE) derivant shown in a formula (I) and a preparation method thereof (n in the formula is an integral number from 0 to 9). The RDG modified DSPE-PEG derivant can increase tumour affinity to meet the requirement of treatment.
Owner:北京中海康医药科技发展有限公司
Core-shell nano particle for covering plant extractive and preparation method thereof
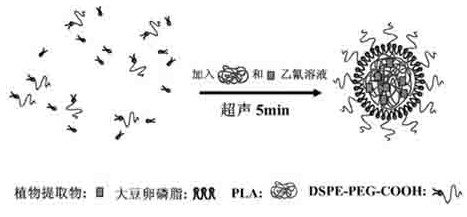
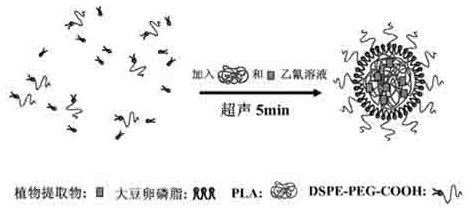
InactiveCN102489229AGood water solubilityReduce releaseMicroballoon preparationMicrocapsule preparationBiotechnologySOY LECITHIN
The invention discloses a core-shell nano particle for covering a plant extractive. The core-shell nano particle comprises a core substance which is the plant extractive; a core membrane is formed by polylactic acid; the polylactic acid covers the plant extractive to be used as an inner core of the core-shell nano particle; single layer of soya bean lecithin is wound around the surface of the polylactic acid and distearoyl phosphatidyl ethanolamine-carboxyl polyethylene glycol is inserted into the soya bean lecithin to be commonly used as a shell of the nano particle. A preparation method is characterized by comprising the following steps of: taking the polylactic acid with a better biocompatibility to be as a carrier; taking the soya bean lecithin and the distearoyl phosphatidyl ethanolamine-carboxyl polyethylene glycol as emulsifiers; and utilizing a one-step ultrasonic synthesizing method to prepare polylactic acid-phospholipid-polyethylene glycol core-shell nano particles for covering the plant extractive.
Owner:SHENZHEN HUACHUANG BIO PHARM TECH CO LTD
Poly(aspartic acid-co-lactic acid)-phosphatidyl ethanolamine graft polymer and preparation method and application thereof
InactiveCN102532533AGood biocompatibilityEasy to acceptPharmaceutical delivery mechanismPharmaceutical non-active ingredientsDrug carrierWater soluble
The invention provides a poly(aspartic acid-co-lactic acid)-phosphatidyl ethanolamine graft polymer, which is characterized in that: the poly(aspartic acid-co-lactic acid)-phosphatidyl ethanolamine graft polymer has a structure shown as a formula (I), wherein n is 15-30, x is 10-120, y is 10-120, z is 10-120, and A is a group with a structure shown as a formula (2). The invention further providesa preparation method of the poly(aspartic acid-co-lactic acid)-phosphatidyl ethanolamine graft polymer and an application thereof to the preparation of a medicinal composition. Nano-micelle of an amphiphilic poly(aspartic acid-co-lactic acid)-phosphatidyl ethanolamine graft polymer can be taken as a water-soluble medicament carrier as well as a fat-soluble medicament carrier, and has a wide application range; and moreover, a medicament effect can be prolonged effectively, and the bioavailability and biological activity are enhanced.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
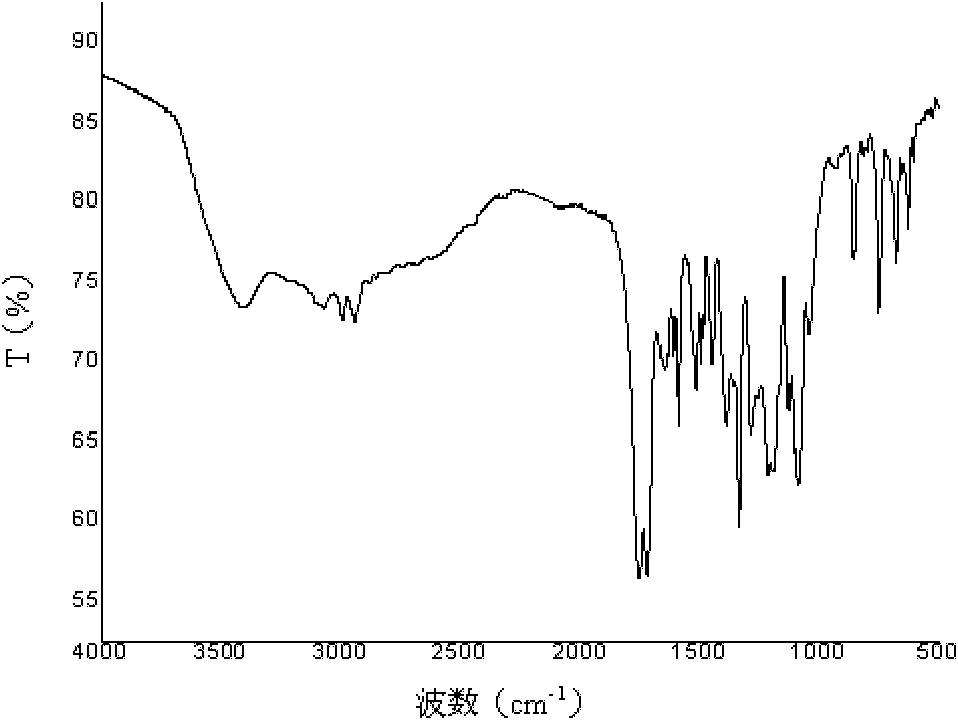
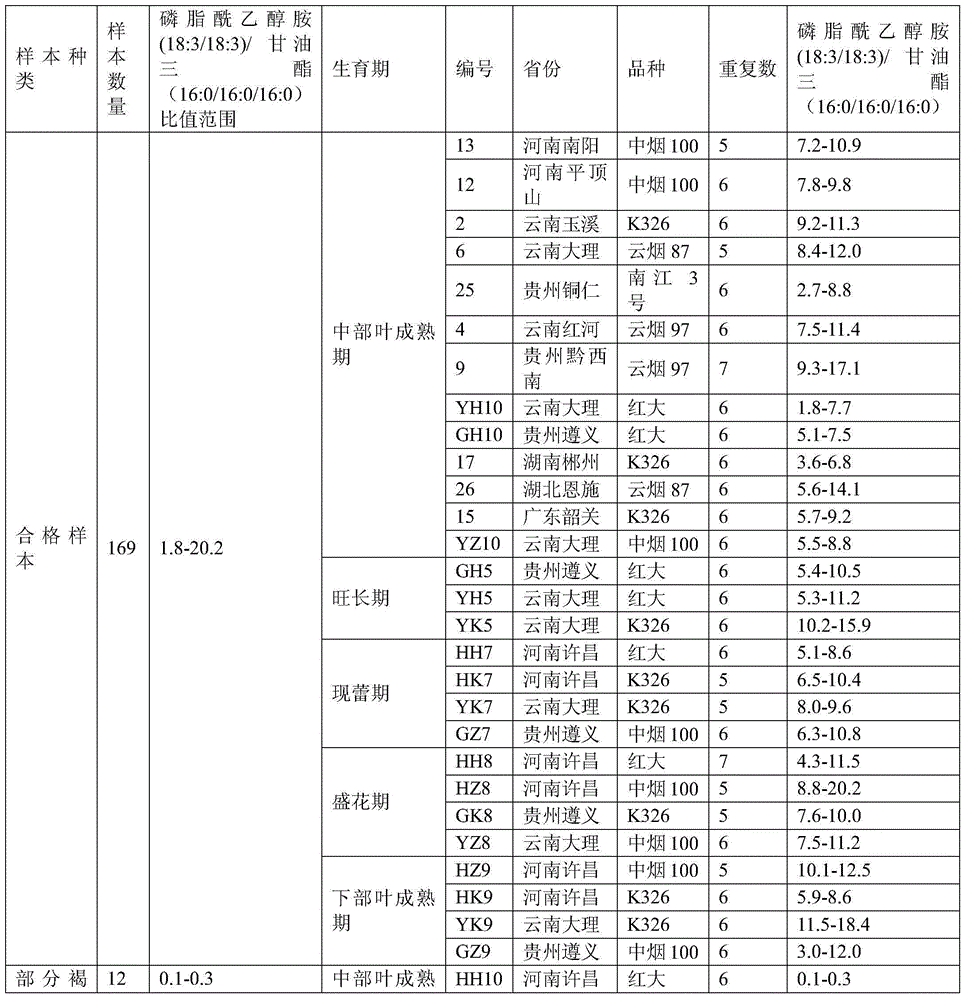
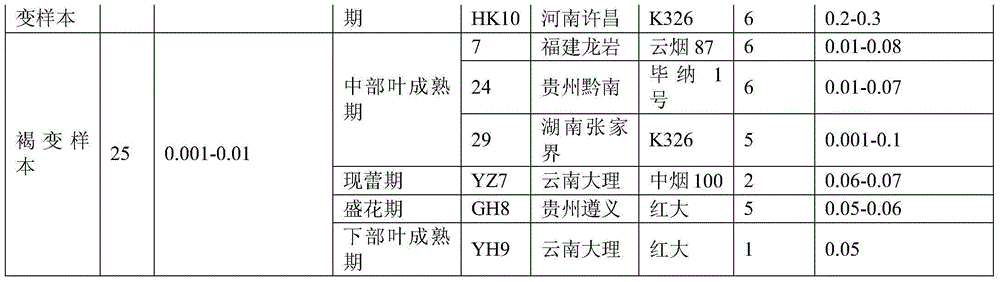
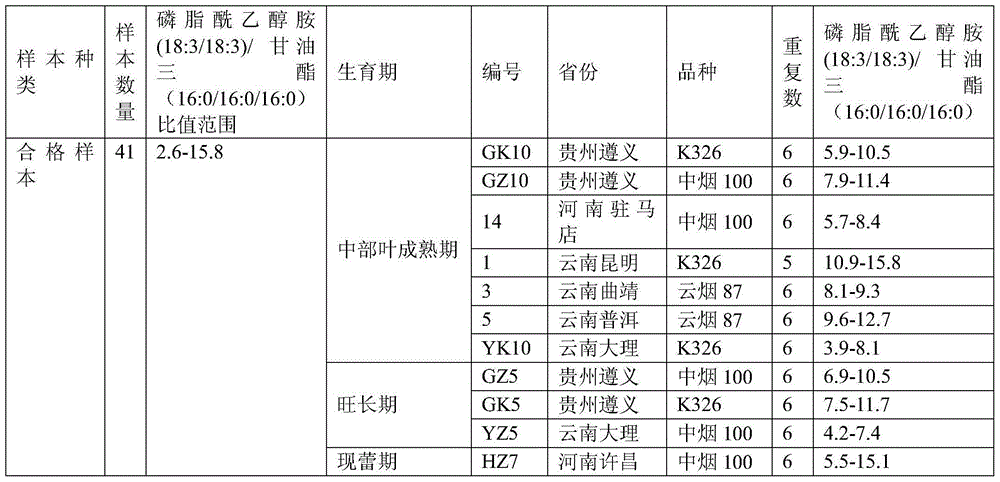
Method for discriminating quality of fresh tobacco leaf sample based on research of metabonomics
The invention provides a method for discriminating the quality of a fresh tobacco leaf sample based on the research of metabonomics. According to the method, the quality of fresh tobacco leaves in the research of metabonomics is discriminated by utilizing the ratio of phosphatidyl ethanolamine (18:3 / 18:3) to triglyceride (16:0 / 16:0 / 16:0). The method is simple and quick to operate and accurate and reliable in results, and can be used for providing reference to analysis of tobacco metabolic characteristics and related researches of tobacco metabolic metabonomics.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
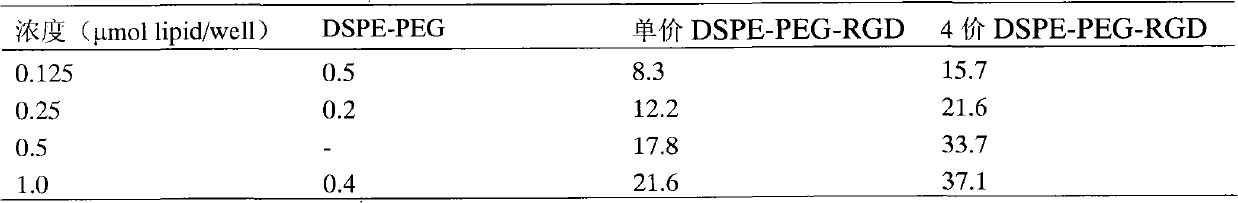
Preparation method of targeting composite nanoparticle
ActiveCN102772367AImprove stabilityExtend cycle timeOrganic active ingredientsPowder deliveryArgininePhospholipid
The invention discloses a preparation method of targeting composite nanoparticle. The invention uses phospholipids, pegylated phospholipids and polyethylene glycol-distearoyl phosphatidyl ethanolamine connected with RGD (arginine-glycine-aspartic acid) as an emulsifier, and uses a binary mixture solvent system of dichloromethane and ethanol to prepare the targeting composite nanoparticle which contains PLGA (poly lactic-co-glycolic acid) as the nucleus and contains the phospholipids and pegylated phospholipids as a shell by one-step emulsification method. The invention uses the hydroxyl camptothecin as a model drug to prepare the RGD targeting phospholipid-polymer composite nanoparticle with high drug loading rate (18.9%) and high entrapment efficiency (94.5%). The phospholipid monolayer and polyethylene glycol hydration layer located on the surface of the targeting composite nanoparticle prepared by the method provided by the invention can significantly reduce protein adsorption and extend the action time in vivo; the RGD connected with the shell of the composite nanoparticle can effectively promote nanoparticles specific binding to tumor cells or tumor new vessels with high expression of integrin, and improve the local drug concentration, so as to enable the targeting composite nanoparticles to have a better anti-tumor effect.
Owner:SUN YAT SEN UNIV
Serum metabolism marker for diagnosis of gestational diabetes mellitus and application thereof
ActiveCN113008972ARaise the possibilityHigh feasibilityComponent separationMaterial analysis by electric/magnetic meansDiabetes mellitusPhysiology
The invention provides a serum metabolism marker for diagnosis of gestational diabetes mellitus and application thereof. Specifically, phosphatidyl ethanolamine PE (O-22: 6_2: 0) and phosphatidyl ethanolamine PE (O-22: 6_3: 0) can be used as a biomarker of gestational diabetes mellitus and can be used for evaluating the illness risk of gestational diabetes mellitus or diagnosing gestational diabetes mellitus. The serum metabolism marker has the advantages of high accuracy and high sensitivity, thereby having important clinical application value.
Owner:南芯芯仪(广州)制造有限公司
Synthesis method of mitochondria-targeting nano triphenylphosphine MoS2 quantum dots
InactiveCN111876144AMild conditionsSimple methodNanoopticsIn-vivo testing preparationsCarboxyl radicalPolyethylene glycol
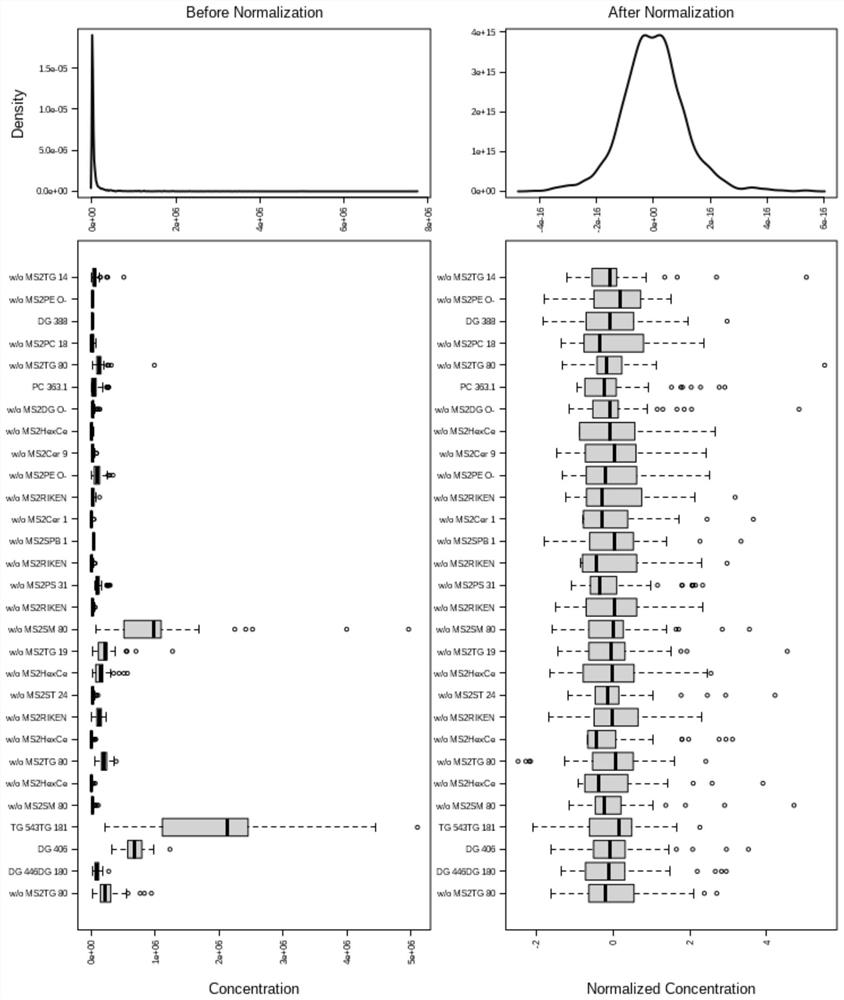
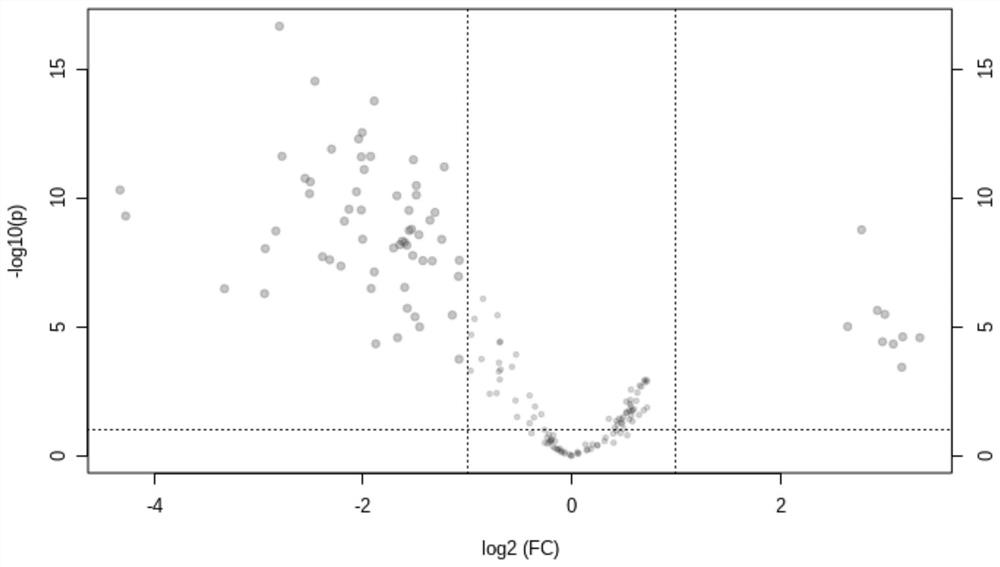
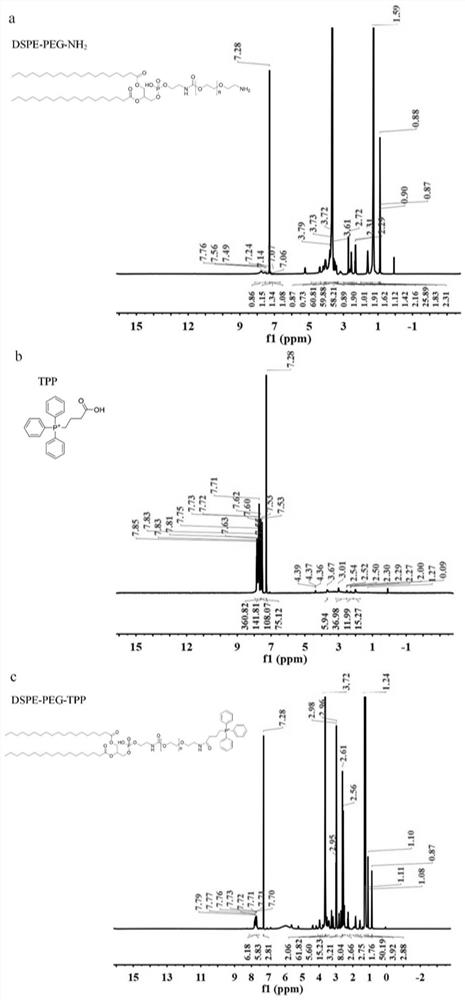
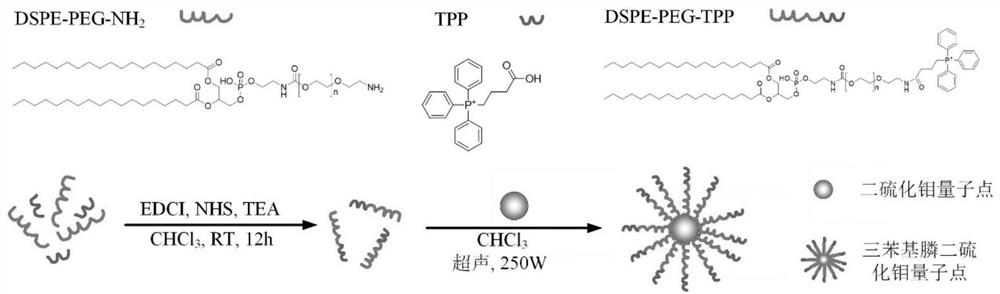
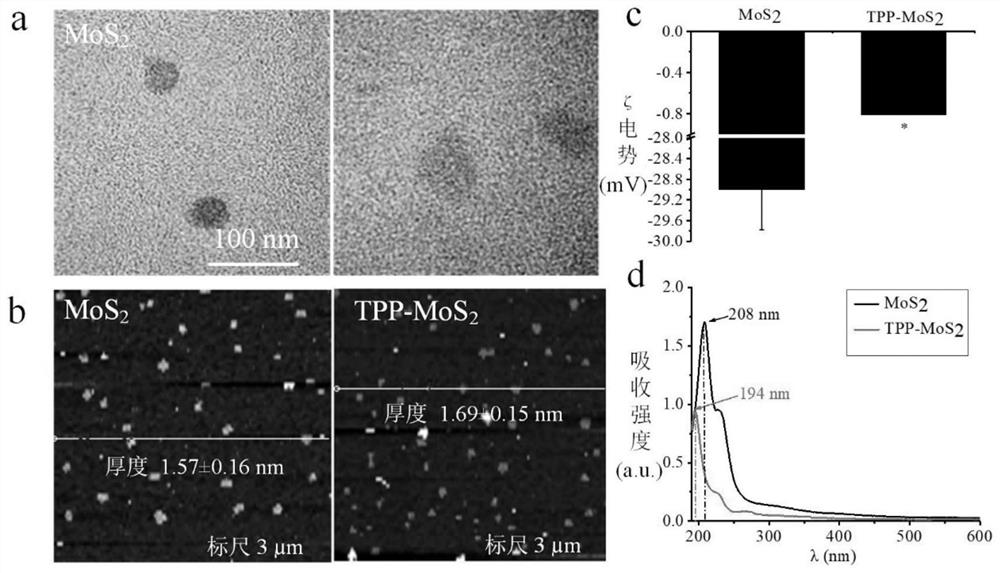
The invention discloses a synthesis method of mitochondria-targeting nano triphenylphosphine MoS2 quantum dots. The preparation method comprises the following steps: firstly, combining amine-modified1 2-distearoyl-sn-glycerin-3phosphoric acid ethanolamine-n-[amino-(polyethylene glycol)-2000] and (3-carboxylpropyl)-triphenylphosphine bromide together through an amino coupling reaction by using a cross-linking agent N-(3-dimethylaminopropyl)-N-ethyl diimine hydrochloride with the length of zero to form DSPE-PEG-GTPP; then, mixing the DSPE-PEG-GTPP and the molybdenum disulfide quantum dot in trichloromethane, wherein the hydrophobic end distearoyl phosphatidyl ethanolamine of the DSPE-PEG-GTPP is connected to the surface of the molybdenum disulfide quantum dot, the (3-carboxylpropyl)-triphenylphosphine bromide end extends outwards, and thus the triphenylphosphine molybdenum disulfide is obtained. The synthesized triphenylphosphine molybdenum disulfide quantum dot can target mitochondria,and a new way is provided for research of mitochondria-related nervous system diseases and mechanisms.
Owner:NANKAI UNIV
Bleomycin hydrocloride lipidosome injection
InactiveCN103040764AComplete formProtection formPowder deliverySaccharide peptide ingredientsMannitolTrehalose
The invention discloses a bleomycin hydrocloride lipidosome injection and a preparation method thereof. The lipidosome injection is prepared by using bleomycin hydrocloride, cholesteryl succinate, distearyl phosphatidyl ethanolamine, polyoxyethylene 40 hydrogenated castor oil, trehalose and mannitol. The bleomycin hydrocloride lipidosome injection disclosed by the invention has the advantage of appropriate particle size, uniform distribution, good stability, higher encapsulation efficiency and lower leaking rate; and the preparation method disclosed by the invention has the advantage of good reproducibility and is suitable for large-scale industrial production.
Owner:海南路易丹尼生物科技有限公司
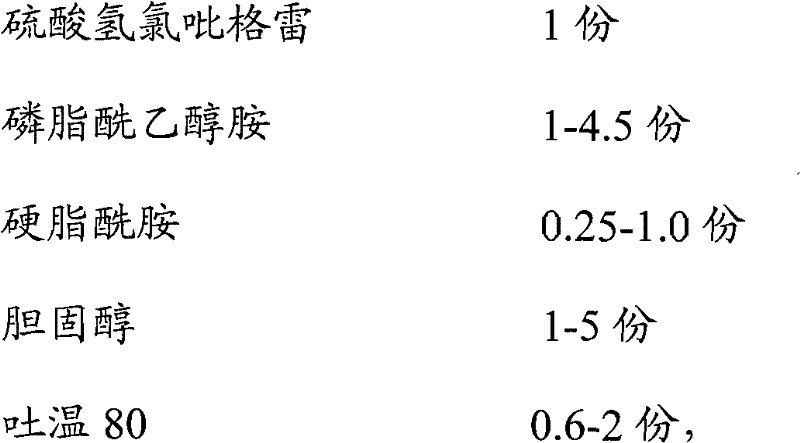
Clopidogrel bisulfate liposome solid preparation
InactiveCN102397253AHigh encapsulation efficiencyImprove stabilityOrganic active ingredientsPill deliverySide effectCholesterol
The invention discloses a clopidogrel bisulfate liposome solid preparation and a preparation method thereof. According to the invention, an active component clopidogrel bisulfate, and phosphatidyl ethanolamine, stearic amide, cholesterol and tween 80 with a certain composition are prepared into a liposome, such that the stability, dissolution rate, and bioavailability of the medicine are substantially improved. Also, the prepared medicine is advantaged in stable and durable effect, and substantial curative effect. With the preparation provided by he invention, product quality of the preparation is improved, and toxic and side-effects are reduced.
Owner:HAINAN LINGKANG PHARMA CO LTD
Nano-composite for early diagnosis and treatment of gastric cancer and preparation method of nano-composite
InactiveCN111135314AImproving Stomach Cancer DiagnosisGood treatment effectPowder deliveryOrganic active ingredientsPolyethylene glycolPhospholipid
The invention discloses a nano-composite for early diagnosis and treatment of gastric cancer and a preparation method of the nano-composite. The nano-composite has a core-shell structure formed by anamphiphilic copolymer, wherein an inner core layer is formed by a distearoyl phosphatidyl ethanolamine fragment, an outer shell layer is formed by a polyethylene glycol fragment, the inner core layerwraps indocyanine green and a hydrophobic gastric cancer chemotherapeutic drug, and the surface of the outer shell layer is connected with RGD short peptides. The nano-composite preparation integratestargeted gastric cancer treatment and fluorescence imaging tracing, targeted aggregation imaging on gastric cancer focus can be implemented, drug release is controlled, the drug concentration of a targeted site is increased, the drug action time is prolonged, and the nano-composite has a wide application prospect in targeted diagnosis and treatment and drug delivery of early gastric cancer.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Environment-friendly cutting fluid and preparation method thereof
InactiveCN105368560AWill not affect healthImprove protectionLubricant compositionSodium bicarbonatePhospholipid
The invention provides an environment-friendly cutting fluid and a preparation method thereof. The cutting fluid includes the components in parts by weight: 6-10 parts of sodium molybdate, 8-12 parts of solid paraffin, 50-60 parts of white mineral oil, 5-10 parts of erucyl amide, 4-8 parts of polyurea, 3-6 parts of phosphatidyl ethanolamine, 3-6 parts of phosphatidyl choline, 5-10 parts of glycerol monostearate, 8-12 parts of glycerol, 4-8 parts of zinc stearate, 2-4 parts of boric acid, 1-2 parts of naphthenic base oil, 1-3 parts of sodium bicarbonate, 1-3 parts of glycerol trioleate, 0.5-1 part of sodium silicate, and 15-25 parts of water. The preparation method comprises the steps: firstly, mixing and heating the components except phosphatidyl ethanolamine, glycerol monostearate and the naphthenic base oil, then adding the remaining three components, continuing to heat, carrying out heat preservation, and cooling to obtain the product. The raw materials used in the cutting fluid and the generated product all can be rapidly degraded and are all harmless to both human bodies and environment, and moreover, all the lubrication, rust protection, cooling and cleaning functions are not inferior to those of other high-quality cutting fluids; and the preparation method is simple in steps, easy to control, prone to industrialization production, and low in production cost.
Owner:SUZHOU JIEDERUI PRECISION MACHINERY
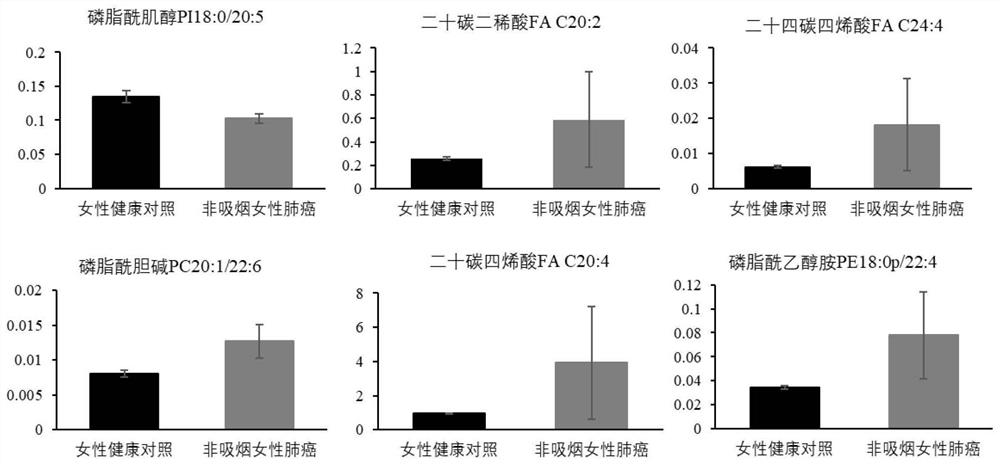
Serum lipid metabolite composition, kit and application
ActiveCN113138275AGood distinctionStrong specificityMaterial analysisPhospholipidPhosphatidyl Cholines
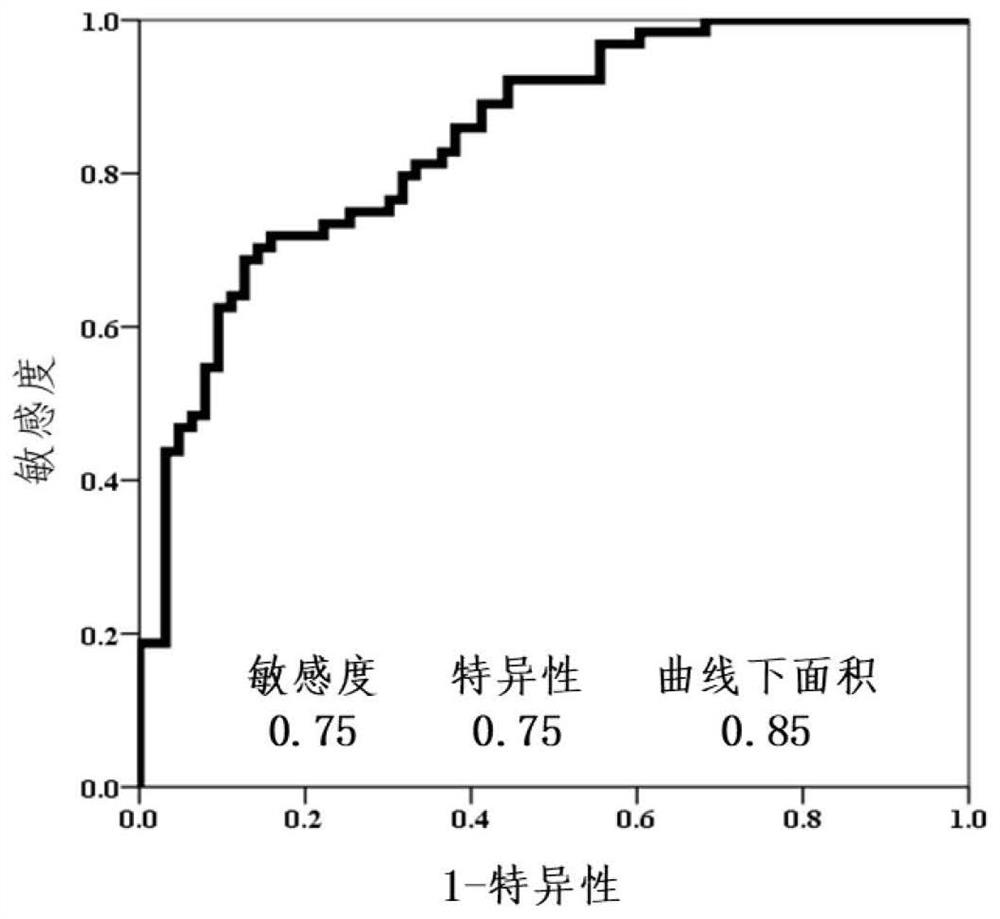
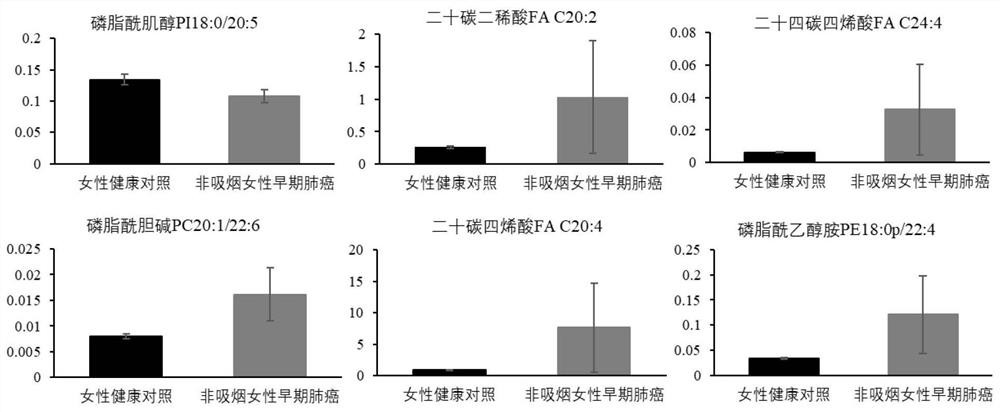
The invention discloses a novel serum lipid metabolite composition and application of the serum lipid metabolite composition as a marker in preparation of a non-smoking female lung cancer diagnostic kit. The serum lipid metabolite composition comprises eicosadienoic acid FA C20:2, eicosatetraenoic acid FA C20:4, tetracosatetraenoic acid FA C24:4, phosphatidylcholine PC 20:1 / 22:6, phosphatidylinositol PI18:0 / 20:5 and phosphatidyl ethanolamine PE18:0p / 22:4. The serum lipid metabolite composition can be used for auxiliary diagnosis of non-smoking female lung cancer, and has the characteristics of low detection cost, good repeatability, and high sensitivity and specificity. Meanwhile, the lipid metabolite composition also has a good effect on diagnosis of early-stage lung cancer of non-smoking females.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Novel aza-crown ether compound as well as cationic liposome, preparation method and application thereof
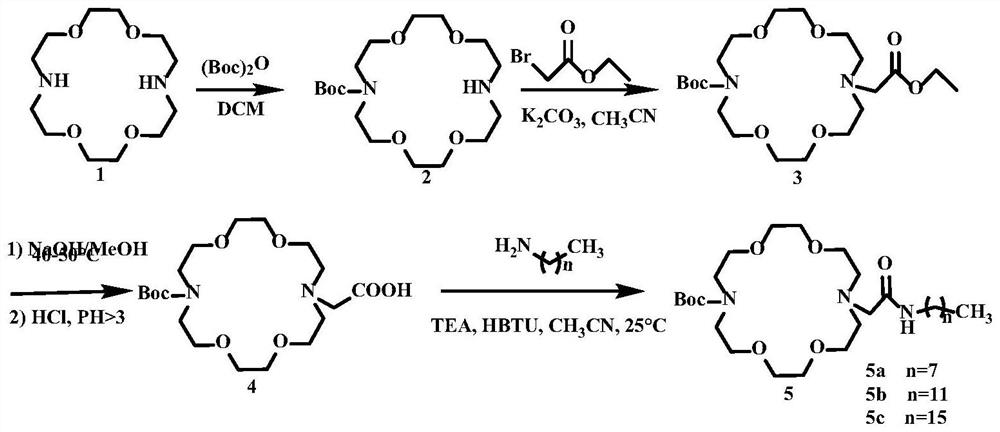
ActiveCN114656422ALow cytotoxicityReduce multidrug resistanceOrganic chemistryPharmaceutical non-active ingredientsCytotoxicityPhospholipid
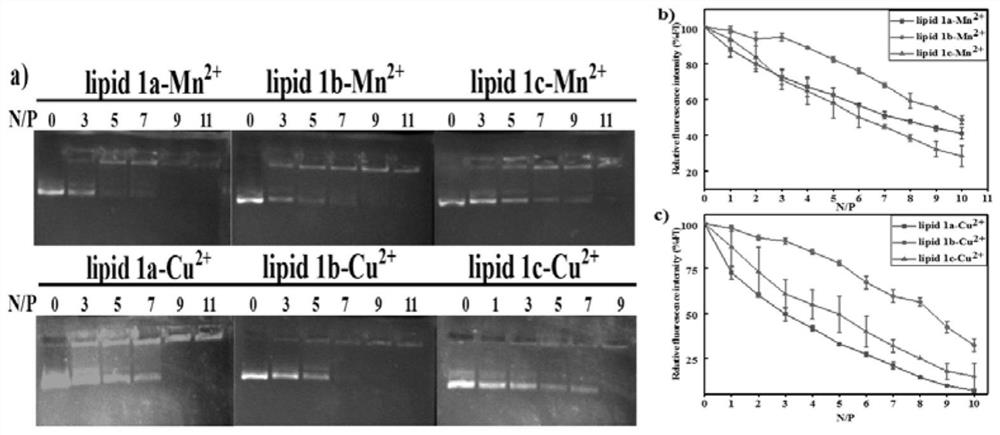
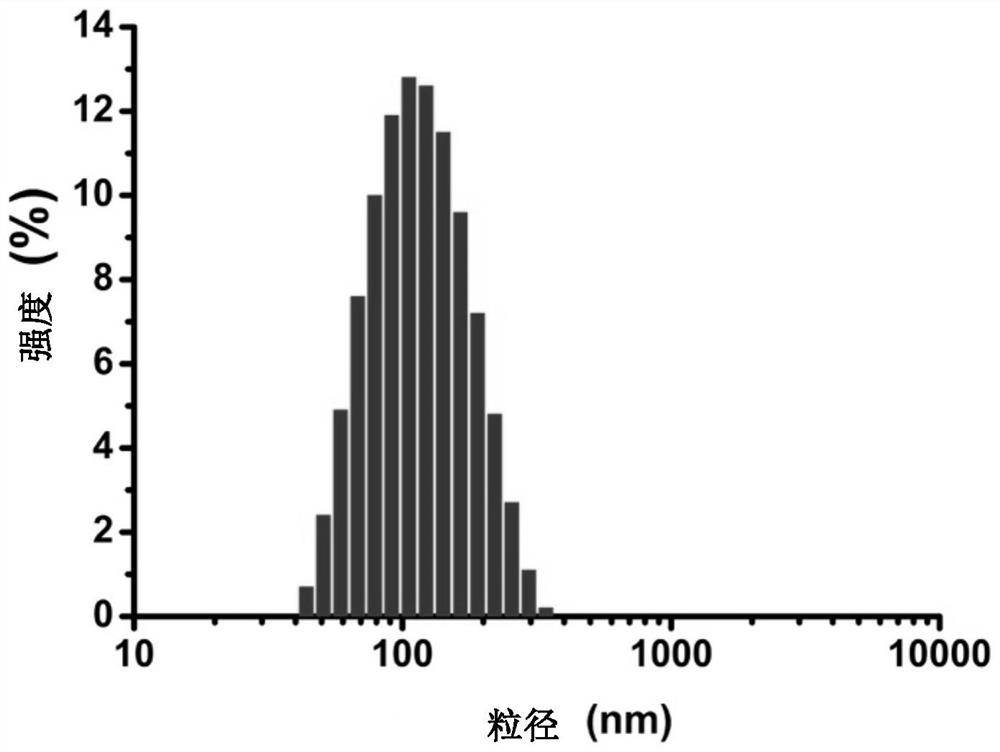
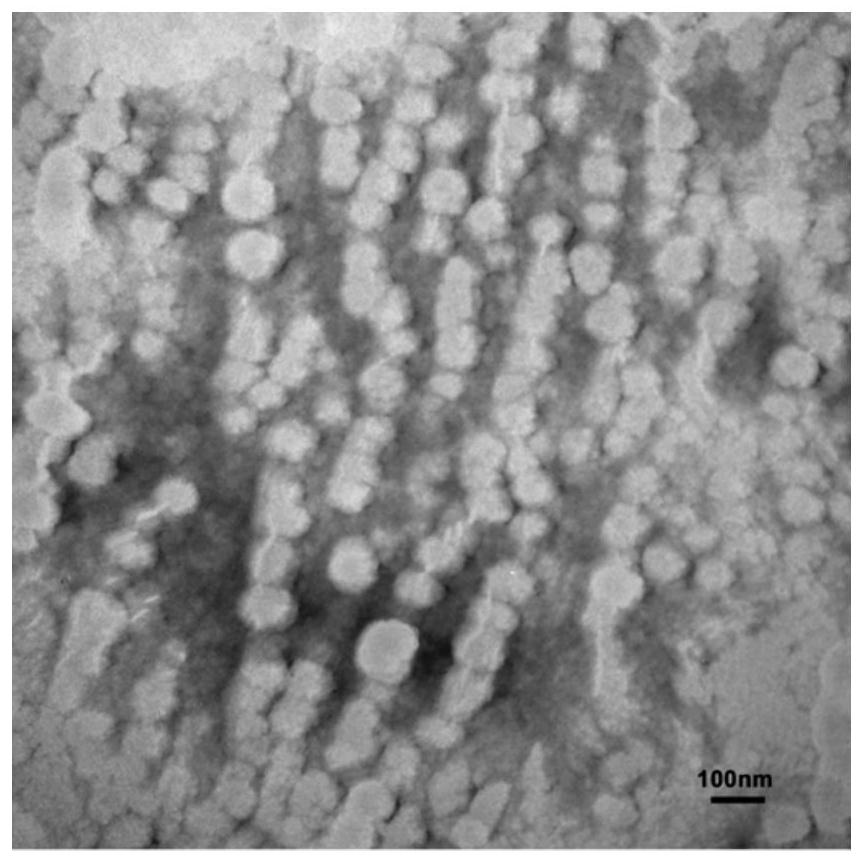
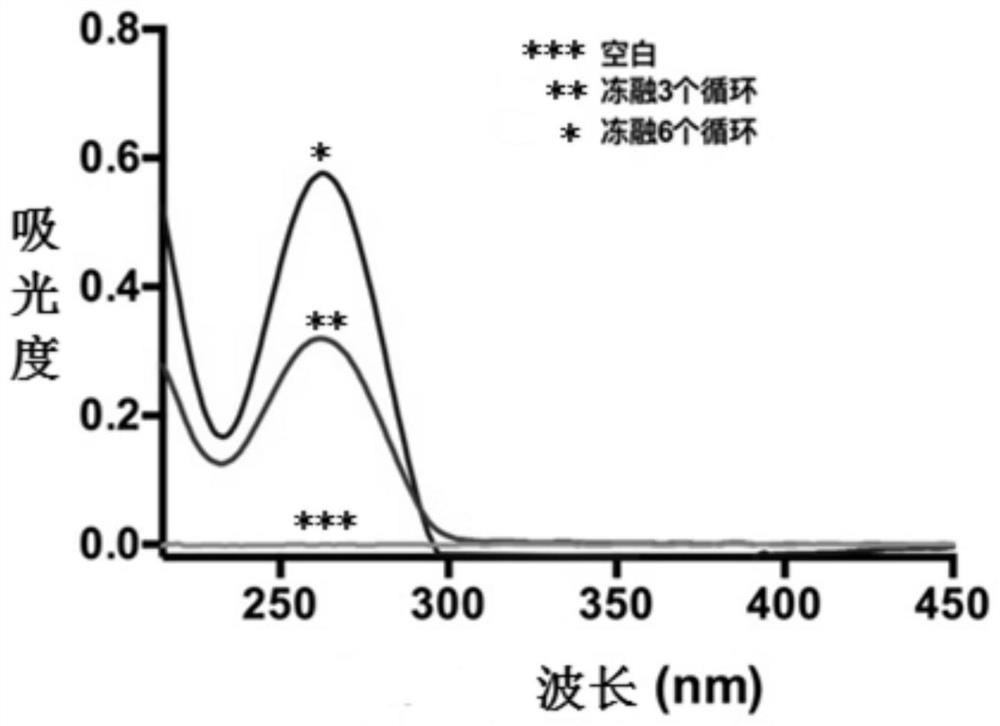
The invention discloses a novel aza-crown ether compound and a cationic liposome, a preparation method and application thereof, and the cationic liposome is prepared by taking diaza 18-crown ether-6 as a raw material, carrying out nucleophilic substitution reaction and hydrolysis reaction, and carrying out HBTU condensation reaction with alkylamine with different carbon chains to obtain the novel aza-crown ether compound. The novel aza-crown ether compound and dienoyl phosphatidyl ethanolamine (DOPE) are assembled to obtain a stable cationic liposome lipid 1-M, and then the cationic liposome and pDNA are subjected to electrostatic interaction to obtain the cationic liposome / pDNA compound. Can be used as a virus vector. Along with the increase of a carbon chain, the cytotoxicity is gradually reduced, and the cell transfection efficiency is gradually improved. A new thought is provided for research of gene vectors, and the application range of the aza-crown ether structure derivative is widened.
Owner:CHONGQING UNIV OF TECH
Nano-drug as well as preparation method and medical application thereof
ActiveCN112190715AHigh drug loadingGood biocompatibilityOrganic active ingredientsMaterial nanotechnologyAptamerCholesterol
The invention belongs to the field of medicine, and particularly relates to a nano-drug. The nano-drug comprises liposome and cholesterol molecule modified aptamer; the cholesterol molecule modified on the aptamer is partially inserted into the liposome, the aptamer is the aptamer of a transferrin receptor, and an acetylcholinesterase heavy activator is wrapped in the liposome, wherein the molar ratio of the liposome to the cholesterol molecule modified aptamer is (5-150): 1; the liposome comprises the following components in parts by molar weight of 25-120 parts of phospholipid, 0.5-30 partsof distearoyl phosphatidyl ethanolamine-polyethylene glycol and 3-45 parts of cholesterol. The invention also relates to the preparation method and application of the nano-drug. The nano-drug disclosed by the invention can be specifically combined with the surface of BBB, effectively penetrates through a blood-brain barrier and releases the drug in the center, and the nano-drug is high in drug loading capacity, good in biocompatibility, high in bioavailability and free of cytotoxicity, and can be used for military protection for treating organophosphorus pesticide poisoning and nerve poisoningagent attack.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Targeted liposome drug delivery system and preparation method and application thereof
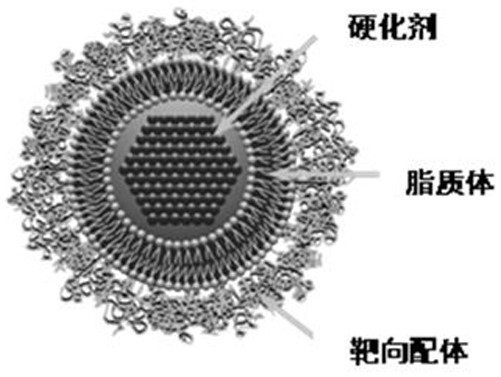
ActiveCN113577300AAvoid rapid degradationProlong the duration of actionSaccharide peptide ingredientsDrug compositionsLiposome membraneLymphatic vessel
The invention discloses a targeted liposome drug delivery system, the drug delivery system is composed of a targeting ligand modified liposome entrapped hardener, and the targeting ligand is hyaluronic acid. The hardener used in the invention is a water-soluble small molecule drug, and the liposome membrane material is prepared from lecithin, cholesterol and distearoyl phosphatidyl ethanolamine-polyethylene glycol-targeting ligand in a mass ratio of (30-32): (7-9): 1. The liposome has a phospholipid bilayer, is self-closed in structure, can entrap a hardener inside the liposome, has good stability, and can prevent water-soluble drugs from being rapidly degraded and reduce the release speed of the drugs. In addition, hyaluronic acid is used for modifying the drug-loaded liposome, so targeted transportation of the drug in lymphangial malformation pathological tissues can be realized.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
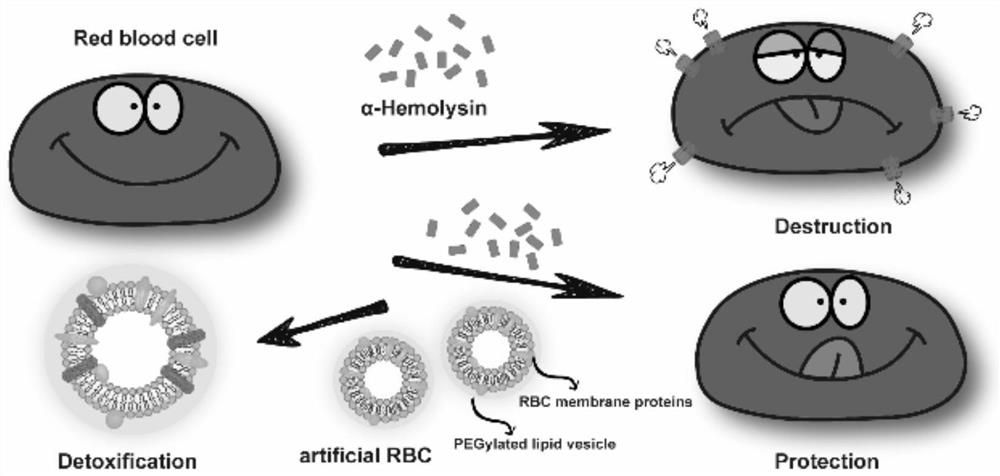
Nano artificial red blood cell and application thereof in preparation of medicine for treating bacterial infection
InactiveCN113041224AAchieve neutralizationAchieve body clearanceAntibacterial agentsPeptide/protein ingredientsCholesterolPolyethylene glycol
The invention belongs to the technical field of biology, and relates to a nano artificial red blood cell and application thereof in preparation of a medicine for treating bacterial infection. The nano medicine is a red blood cell membrane protein liposome formed by integrating red blood cell membrane protein into an artificial lipid membrane, wherein the lipid membrane is composed of phosphatidylcholine, polyethylene glycol distearoyl phosphatidyl ethanolamine and / or cholesterol. The nano medicine adsorbs pore-forming toxins on the surface by simulating the red blood cell, neutralizes the toxicity of the toxins, and realizes anti-virulence factor treatment, thereby enhancing the effect of anti-bacterial infection treatment, especially drug-resistant bacterial infection.
Owner:FUDAN UNIV
Nisoldipine liposome solid preparation
InactiveCN102406608AHigh encapsulation efficiencyImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
The invention discloses a nisoldipine liposome solid preparation and a preparation method thereof. The liposome is prepared from an active component nisoldipine and specifically combined phosphatidyl ethanolamine, phosphatidylcholine distearate, acetyl cholesterol and tween 80, so that the stability, solubility and bioavailability of the medicament can be greatly improved, the action is smooth and durable, and the curative effect is remarkable. According to the preparation, the product quality of the preparation is increased, and toxic and side effects are reduced.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Tetrandrine-loaded liposome preparation as well as preparation method and application thereof
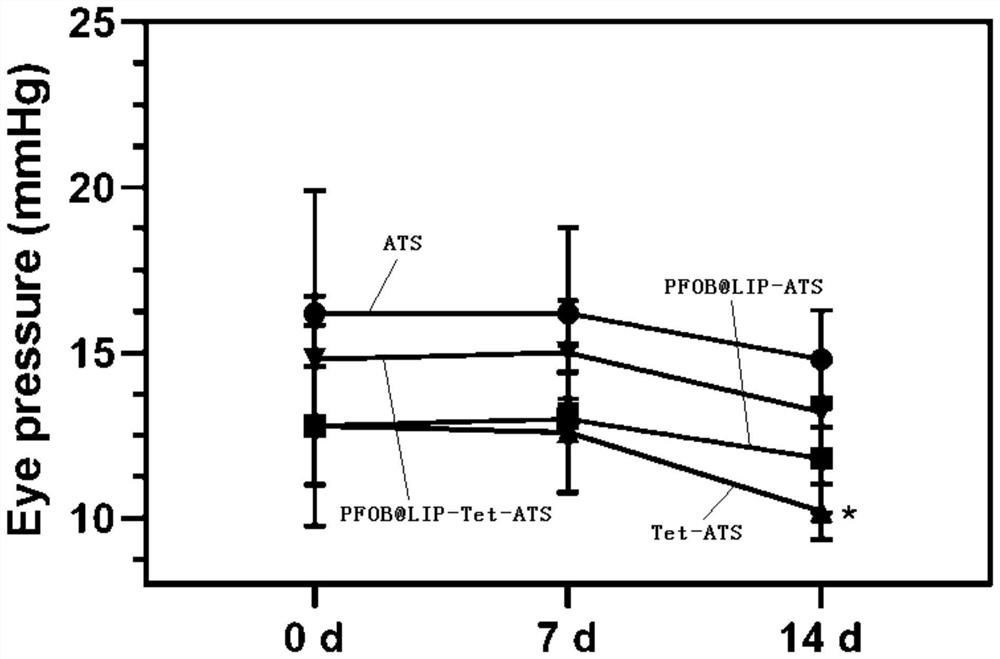
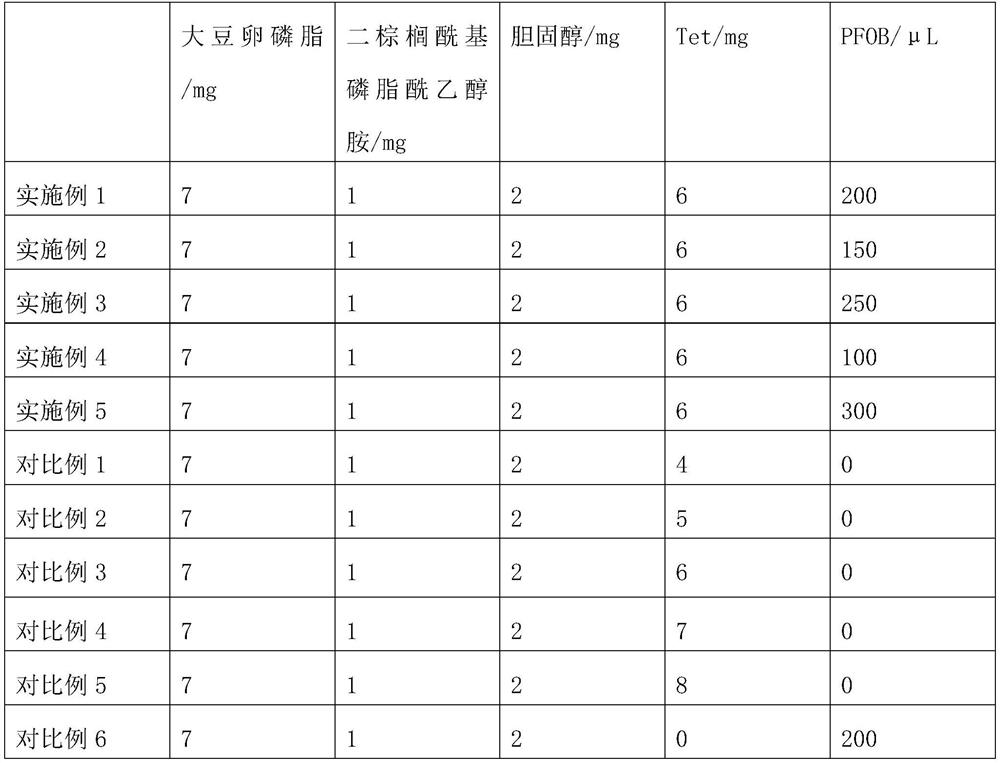
ActiveCN112826795AHigh encapsulation efficiencyAvoid unevennessOrganic active ingredientsSenses disorderCholesterolIntraocular pressure
The invention relates to the field of pharmaceutical preparations, and discloses a tetrandrine-loaded liposome preparation, which is prepared from the following raw materials: soya bean lecithin, dipalmitoyl phosphatidyl ethanolamine, cholesterol, tetrandrine and perfluorobromooctane, the mass ratio of the sum of the mass of the soybean lecithin and the dipalmitoyl phosphatidyl ethanolamine to the mass of the cholesterol to the tetrandrine is 4: 1: (2.5-3.5). The preparation method of the tetrandrine-loaded lipidosome preparation comprises the following steps: dissolving soybean lecithin, dipalmitoyl phosphatidyl ethanolamine, cholesterol and tetrandrine in a solvent, carrying out rotary evaporation to obtain a uniform thin liquid film, hydrating the thin liquid film, adding perfluorobromooctane, carrying out ultrasonic oscillation in an ice bath, centrifuging, collecting a precipitate, and obtaining the tetrandrine-loaded liposome preparation. The tetrandrine-loaded liposome preparation is applied to preparation of medicaments for treating dry eye diseases, has small influence on intraocular pressure, and greatly improves the entrapment efficiency and drug loading rate of tetrandrine.
Owner:CHONGQING MEDICAL UNIVERSITY
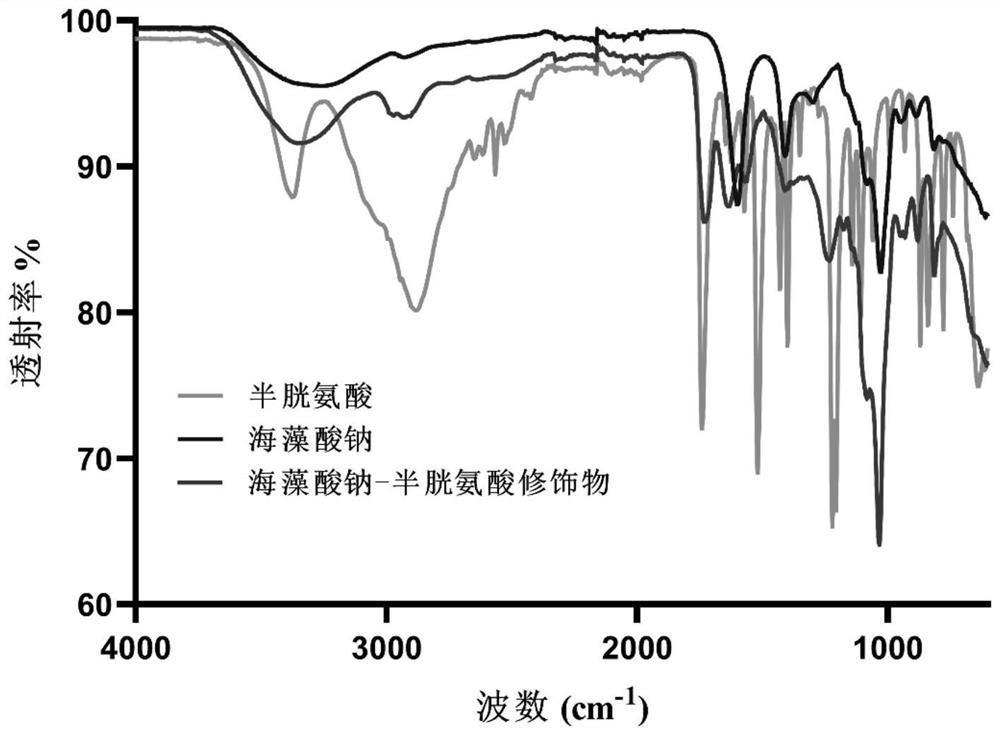
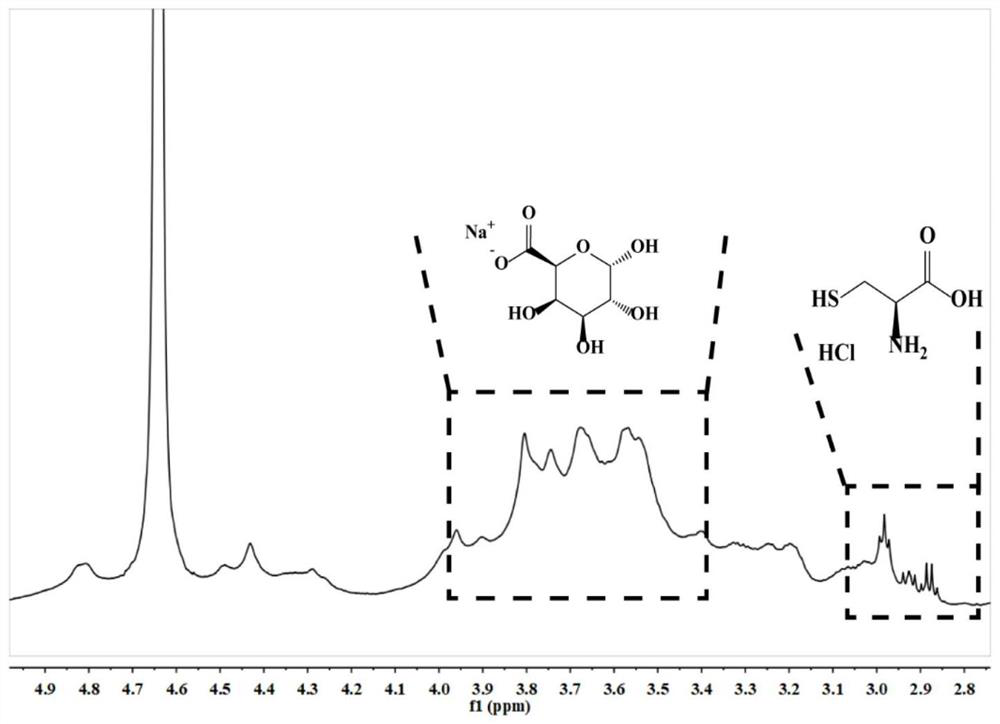
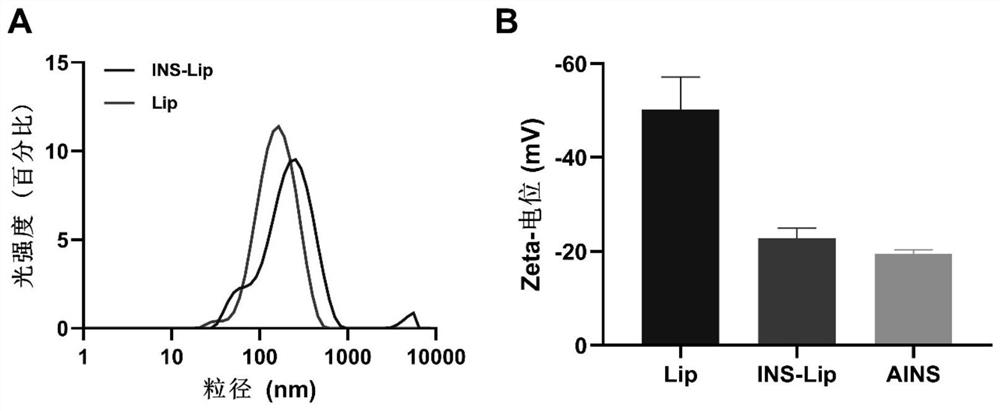
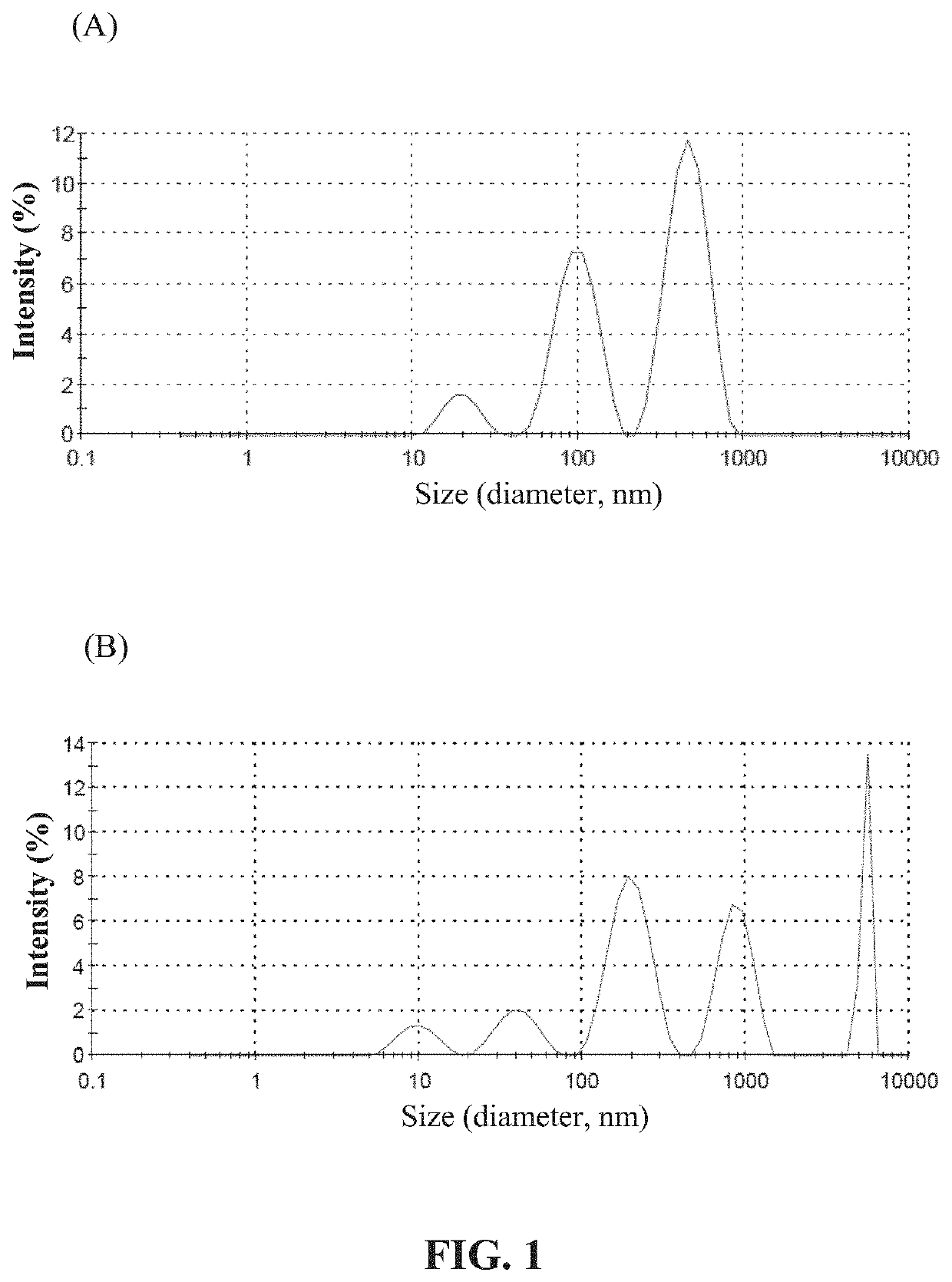
Oral insulin liposome hydrogel and application thereof
PendingCN114767837AGuaranteed functionImprove protectionPeptide/protein ingredientsMetabolism disorderCholesterolPhospholipid
The invention discloses oral insulin liposome hydrogel and application thereof, and belongs to the technical field of insulin pharmacy. The oral insulin liposome hydrogel comprises an insulin-loaded liposome and a hydrogel with a core-shell structure formed by modified sodium alginate, the core is the insulin-loaded liposome, and the shell is a hydrogel framework formed by the modified sodium alginate; the insulin-loaded liposome is formed by mixing an insulin compound, phosphatidyl ethanolamine and cholesterol in an organic solvent, the particle size of the insulin-loaded liposome is 100-200 nm, and the PD I value of the insulin-loaded liposome is 0.1-0.4; the modified sodium alginate is obtained by reacting sulfydryl-containing amino acid with sodium alginate. Comprising the oral insulin liposome hydrogel prepared by the invention can be orally administered to a patient, and compared with injection, the oral insulin liposome hydrogel has the advantages that the pain of the patient is small, repeated administration is not needed, and the blood sugar control is stable; the hypoglycemic effect is good, the hypoglycemic duration is long, the effect is remarkable, and the bioavailability of insulin is high.
Owner:HEFEI UNIV OF TECH
Emulsified liposome composition and preparation process thereof
InactiveUS20200107999A1Improve permeabilityPromote absorptionCosmetic preparationsToilet preparationsPhospholipidBULK ACTIVE INGREDIENT
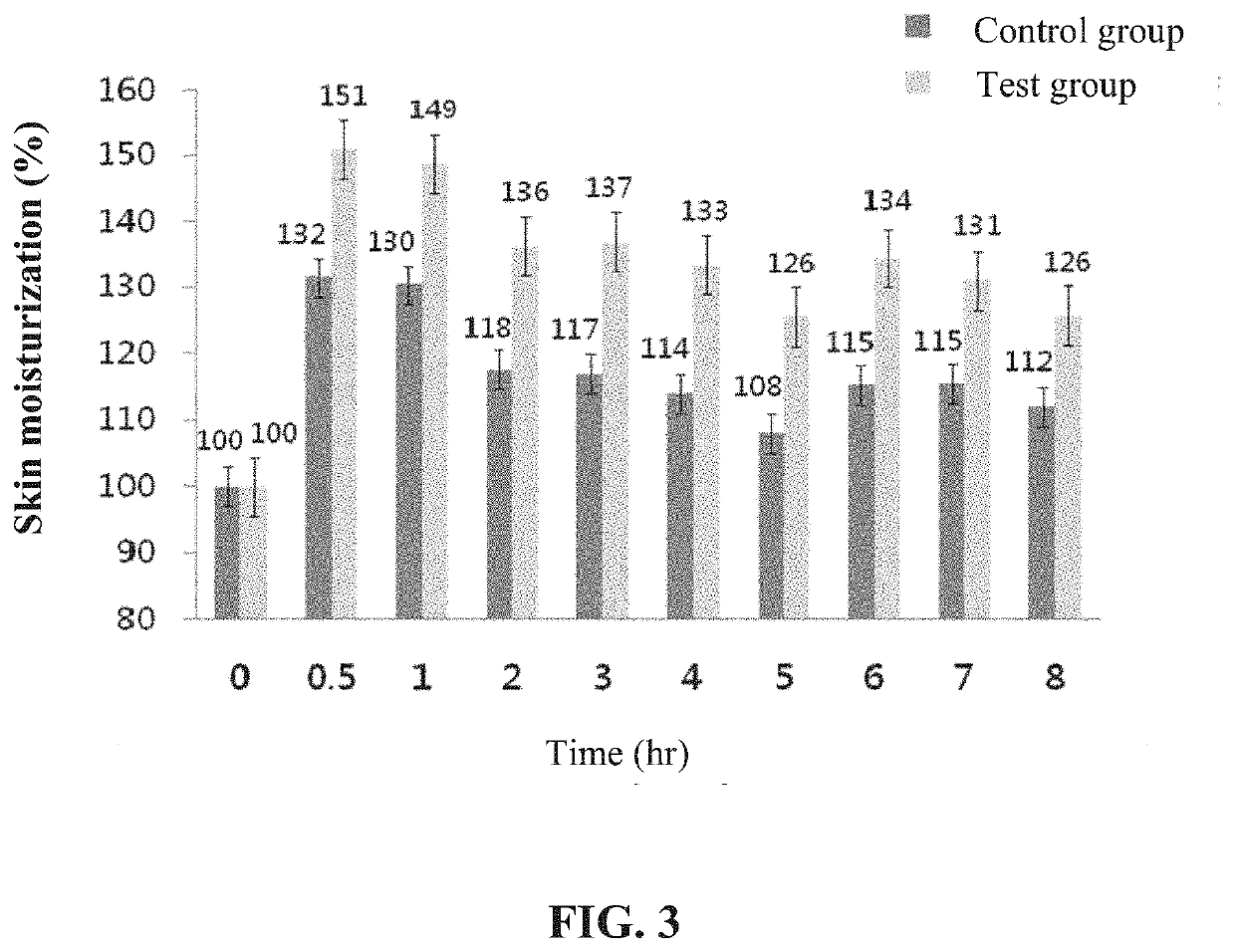
The present disclosure relates to an emulsified liposome composition and a method for preparing the emulsified liposome composition. The emulsified liposome composition includes a phospholipid, wherein the phospholipid includes a phosphatidyl ethanol amine and a phosphatidylcholine, and a weight ratio of the phosphatidyl ethanol amine to the phosphatidylcholine is 3-6:1. Through the emulsified liposome composition of the present disclosure, the liposome has a small particle diameter, so that it can penetrate into the deep layer of the skin quickly and deeply. Through the moisturizing or anti-oxidant active ingredient encapsulated in the emulsified liposome composition, the emulsified liposome composition has skin moisturizing and anti-oxidation effect, and can be widely used in products regarding maintenance, make-up, and sun-checking.
Owner:TCI CO LTD
Molybdenum oxide nanosheet plugging hollow mesoporous silicon nano material, and preparation method and application thereof
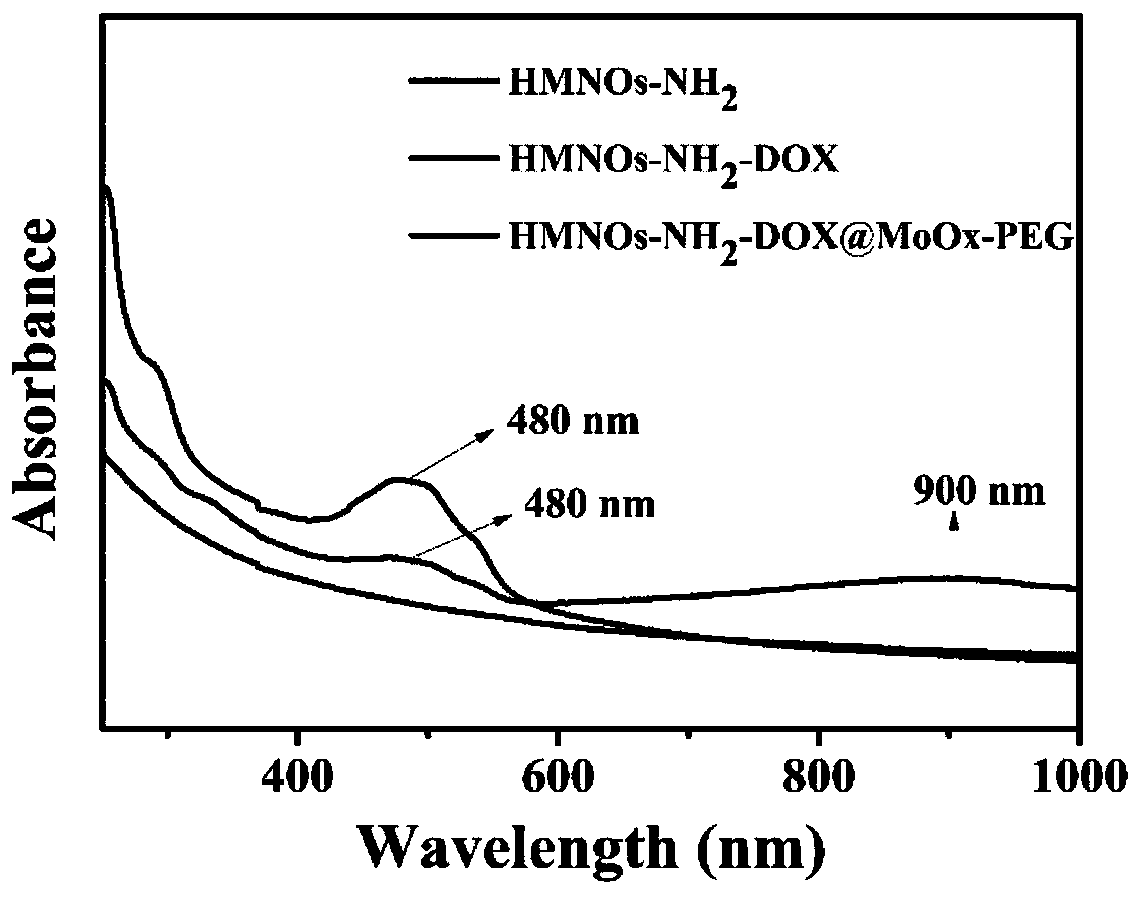
ActiveCN110917172AAchieve controlled releaseImprove stabilityOrganic active ingredientsDrug photocleavagePolyethylene glycolEthanolamines
The invention relates to a molybdenum oxide nanosheet plugging hollow mesoporous silicon nano material, and a preparation method and application thereof. The molybdenum oxide nanosheet plugging hollowmesoporous silicon nano material is obtained by plugging hollow mesoporous silicon by polyethylene glycol modified distearoyl phosphatidyl ethanolamine mPEG-DSPE. The preparation method is simple tooperate, the experimental conditions are easy to control, and the method has a prospect of industrial implementation.
Owner:DONGHUA UNIV
METHOD FOR INHIBITING TUMOR GROWTH THROUGH RNA-INTERFERENCE USING LIPOSOMALLY ASSOCIATED CDC20 siRNA
InactiveUS20160010088A1Improve efficiencyLow toxicitySpecial deliveryFermentationLipid formationMelanoma
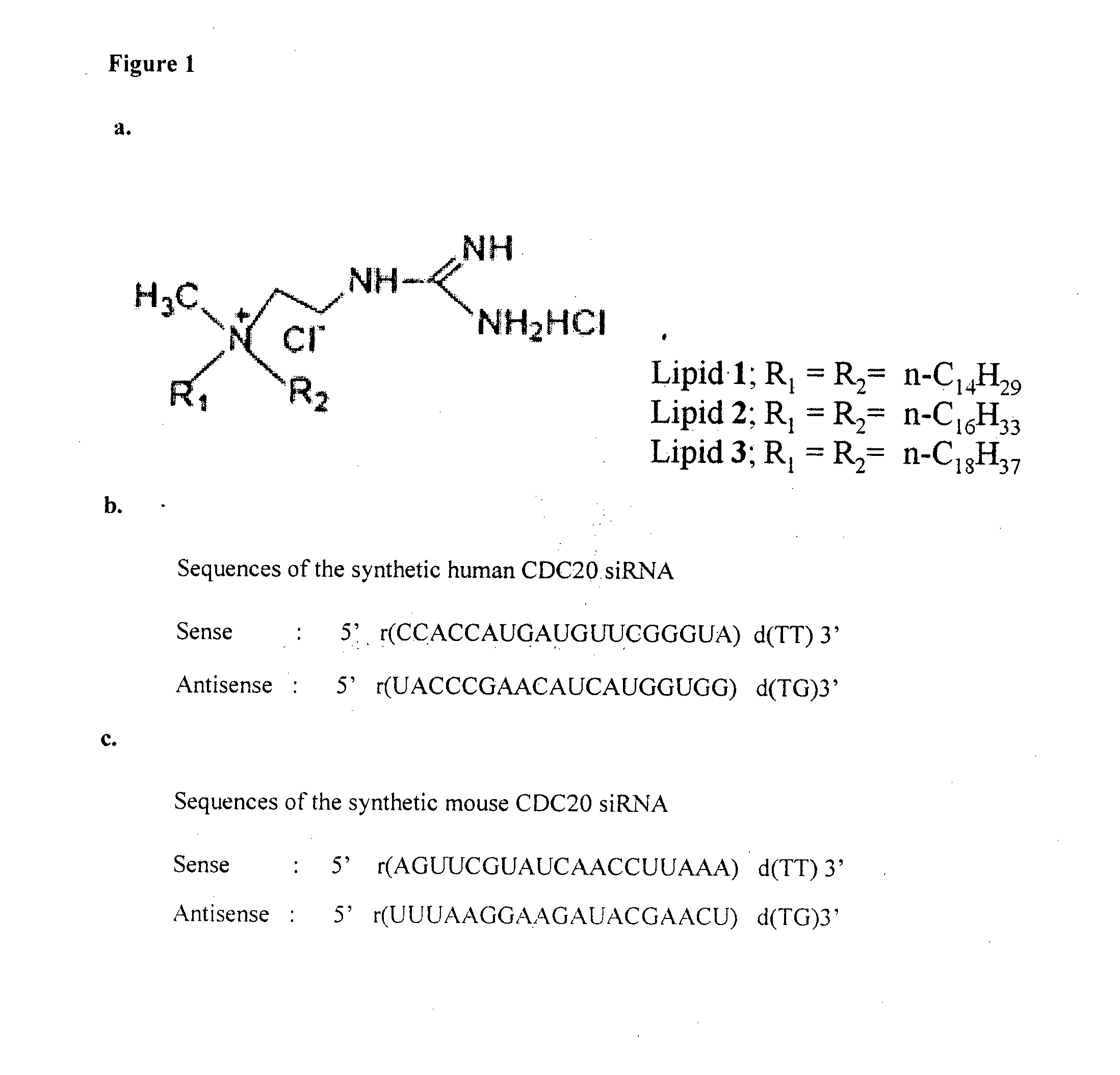
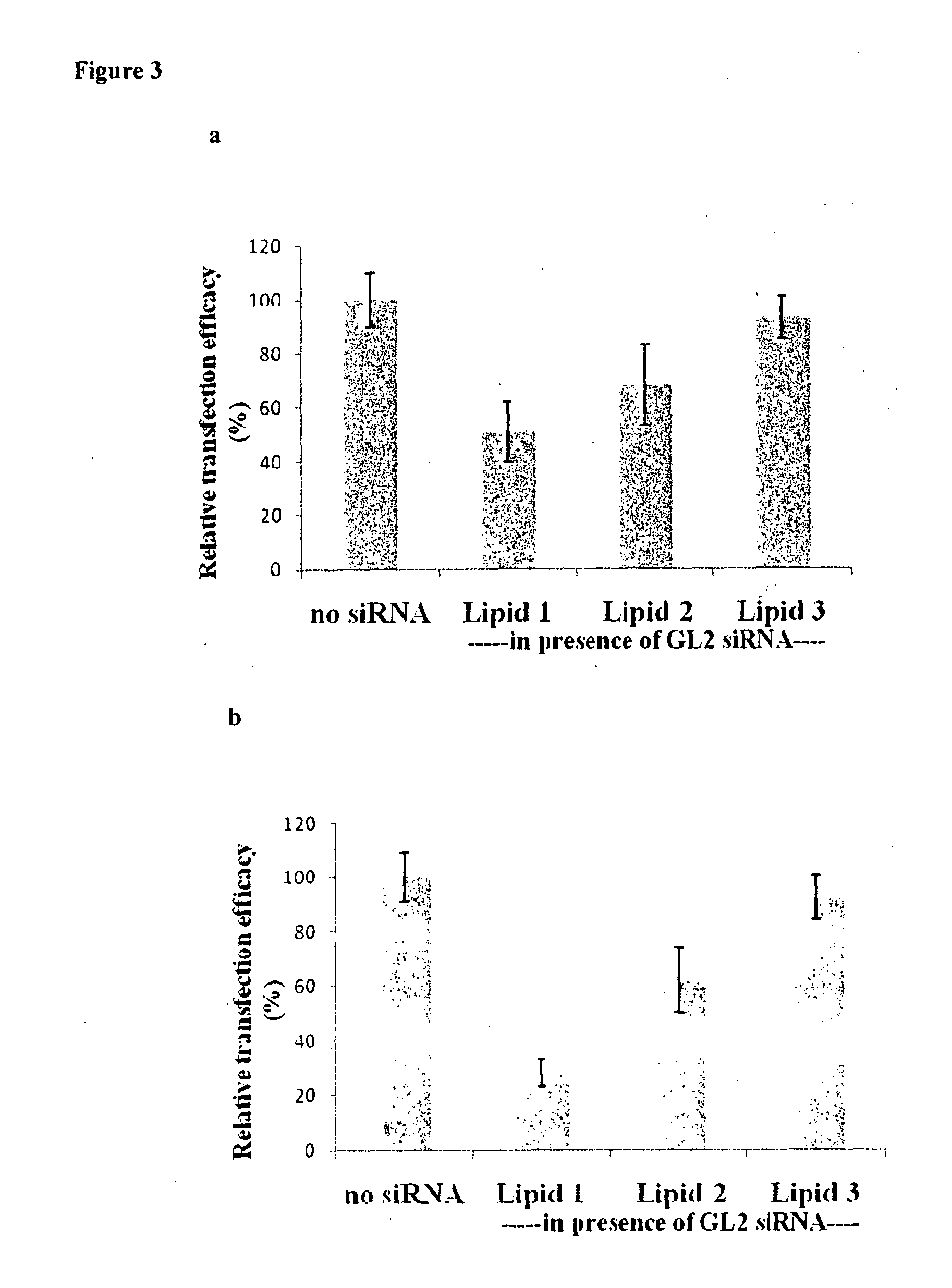
Liposomal compositions comprising of liposomes of guanidinylated cationic amphiphiles as the main lipid and cholesterol / 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) / aminopropyl polyethyleneglycol carbamyl-distearoylphosphatidyl-ethanolamine (DSPE-peg-NH2) as co-lipids are described. These liposomal compositions containing encapsulated or electrostatically complexed therapeutic small interfering RNAs (siRNAs) against Cdc20, a key cell cycle regulator, inhibit solid tumor growth and melanoma tumor growth on lung in C57BL / 6J mice.
Owner:COUNCIL OF SCI & IND RES
Personal care product
InactiveCN111417380AImprove sensory propertiesAdjust and balance the lookCosmetic preparationsHair cosmeticsVegetable oilMedicine
A personal care formulation comprises an emulsifier, a vegetable oil and optionally a texturizer, wherein the emulsifier contains an Acetone Insoluble (AI) component containing at least Phosphatidyl Choline (PC) and Phosphatidyl Ethanolamine (PE), wherein the emulsifier has a weight ratio R of at least 5.0.
Owner:CARGILL INC
Feed additive for enriching phosphatidyl ethanolamine DHA in eggs, and preparation method and application thereof
ActiveCN113974017AEnrichment stabilityStable structureFood processingAnimal feeding stuffBiotechnologySoybean Phospholipids
The invention provides a feed additive capable of enriching phosphatidyl ethanolamine DHA in eggs. The feed additive contains directionally purified soybean phospholipids, wall-broken schizochytrium limacinum powder, selenium yeast and cysteine. Wherein the directionally purified soybean phospholipid is rich in phosphatidyl ethanolamine soybean phospholipid, the schizochytrium limacinum provides DHA, selenium yeast and cysteine which can generate a certain content of glutathione peroxidase in chicken bodies, full combination of phosphatidyl ethanolamine and DHA is guaranteed, the structure and content of phosphatidyl ethanolamine DHA are stabilized, and phosphatidyl ethanolamine DHA is stably enriched in eggs. The directionally purified soybean phospholipid, the wall-broken schizochytrium limacinum powder, the selenium yeast and the cysteine are powder and are easy to mix uniformly, so that the preparation method is different from the prior art in which oily substances such as schizochytrium limacinum oil are adopted, and the preparation difficulty is reduced.
Owner:HUNAN AGRICULTURAL UNIV
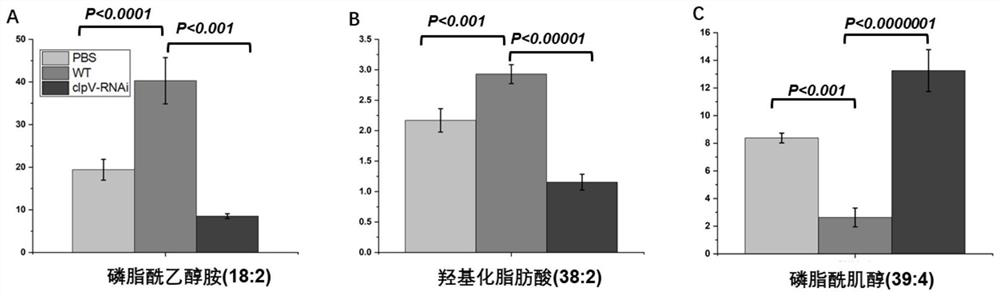
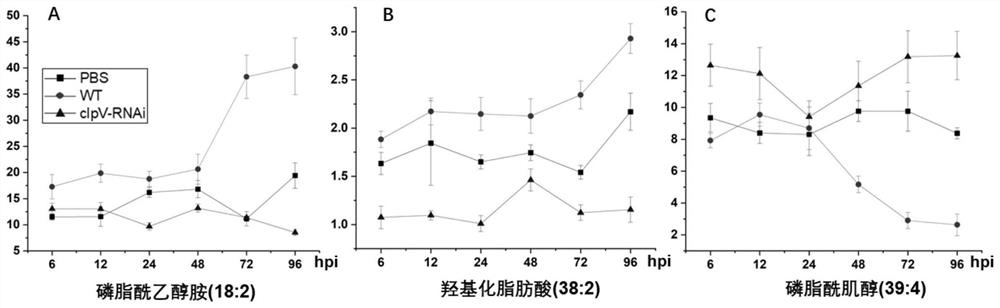
Combined metabolic marker and detection kit for judging genetic modification effect of pathogenic bacteria of visceral white-spot disease of epinephelus coioides
The invention discloses a combined metabolic marker and a detection kit for judging the genetic modification effect of pathogenic bacteria of visceral white-spot disease of epinephelus coioides, and relates to lipid metabolites such as phosphatidyl ethanolamine (18:2), hydroxylated fatty acid (38:2) and phosphatidylinositol (39:4) in spleen samples of epinephelus coioides as combined markers, and the method is used for judging the effect of silencing of the pseudomonas proteiniflora virulence gene clpV causing the grouper visceral white-spot disease on the grouper. The invention also relates to a kit for judging the gene modification effect. The method comprises the following steps: detecting the relative concentration of lipid metabolites in a spleen sample of grouper infected by pseudomonas proteinus, calculating a combined marker variable P based on a binary logistic regression equation, and determining a cut point value, and judging the condition of the pathogenic bacteria of the visceral white-spot disease of the grouper after genetic modification. The kit has the characteristics of high sensitivity, high detection efficiency, low cost and good repeatability, and has a good application prospect.
Owner:JIMEI UNIV
Functional vinblastine lipidosome and application thereof
ActiveCN107875123AOrganic active ingredientsMacromolecular non-active ingredientsPolythylene glycolPhospholipid
The invention discloses a functional vinblastine lipidosome and an application thereof. The invention provides the vinblastine lipidosome which is prepared by the following steps: modifying a lipidosome with a functional material transferrin receptor combined peptide-polyethylene glycol-distearyl phosphatidyl ethanolamine and stearylated octaarginine to obtain a modified lipidosome; and then entrapping vinblastine to the modified lipidosome to obtain the functional vinblastine lipidosome. Experiments verify that the invention synthesizes the functional material transferrin receptor combined peptide-polyethylene glycol-distearyl phosphatidyl ethanolamine (NHS-PEG2000-DSPE) and modifies the vinblastine lipidosome with a targeting material stearylated octaarginine (stearyl-R8) so as to obtainthe functional vinblastine lipidosome capable of bestriding the blood brain barrier and killing glioma and stem cells thereof.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com