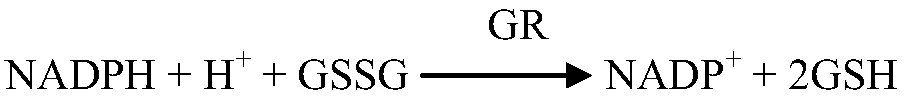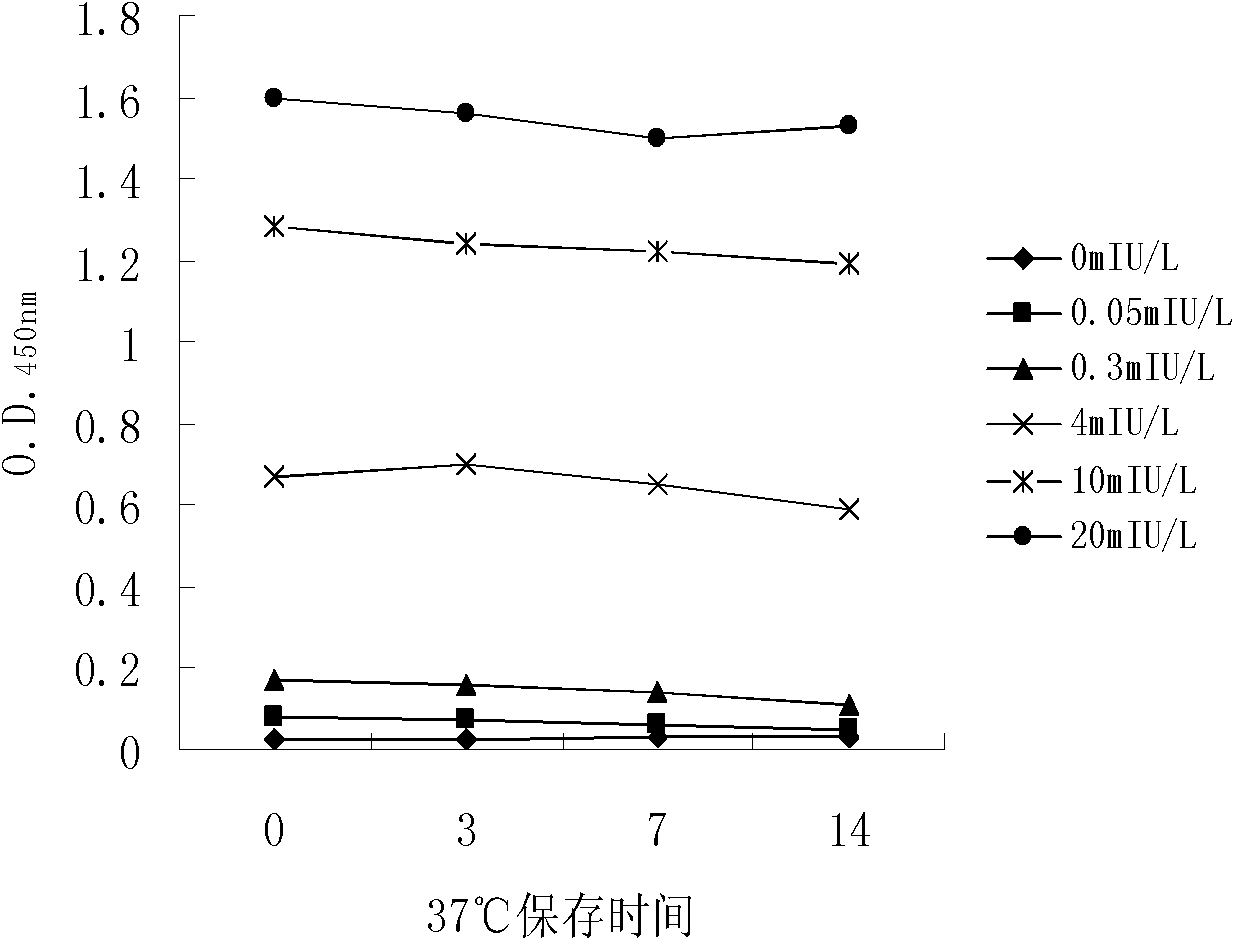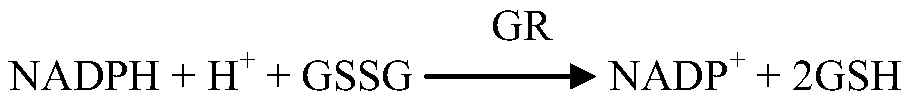Patents
Literature
63results about How to "Meet the needs of clinical testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enzyme-promoting chemiluminiscence substrate using alkaline phosphatase
ActiveCN104990912AHigh strengthLow costChemiluminescene/bioluminescenceBiological testingFluorescencePerformance index
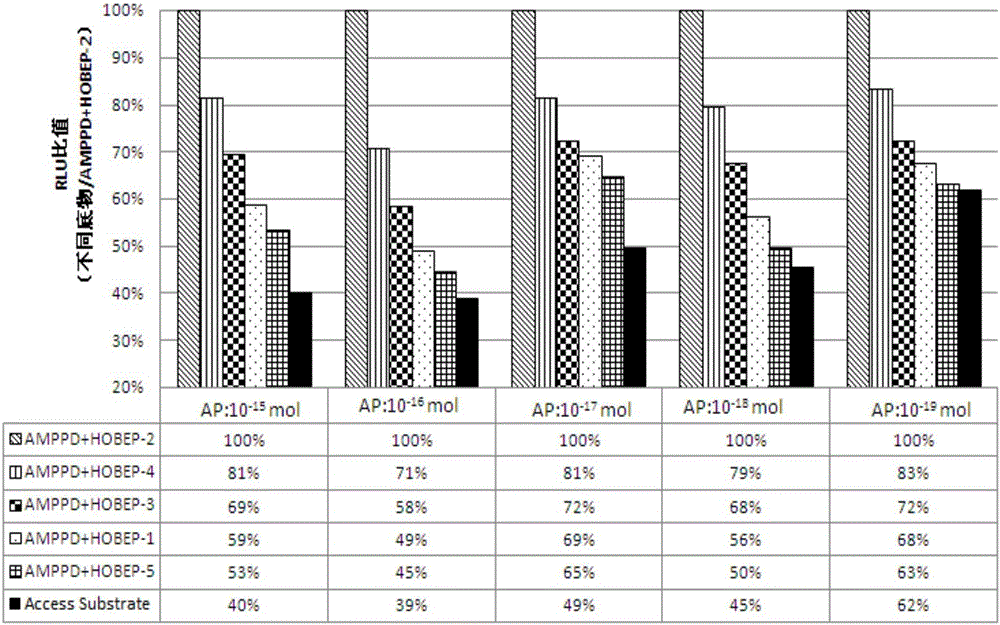
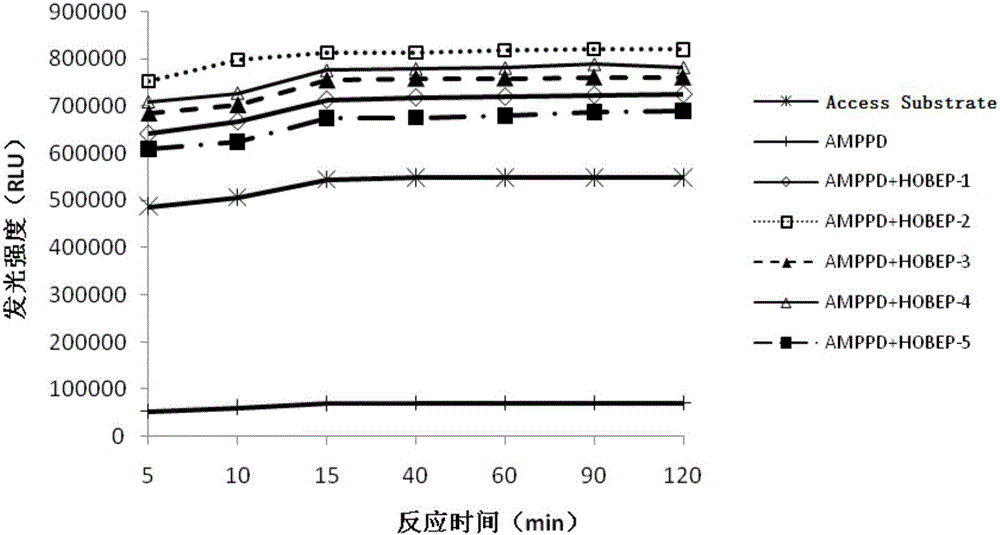
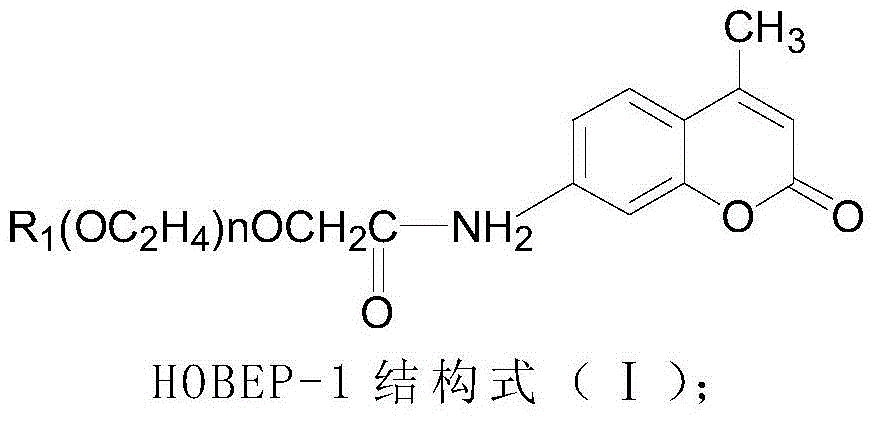
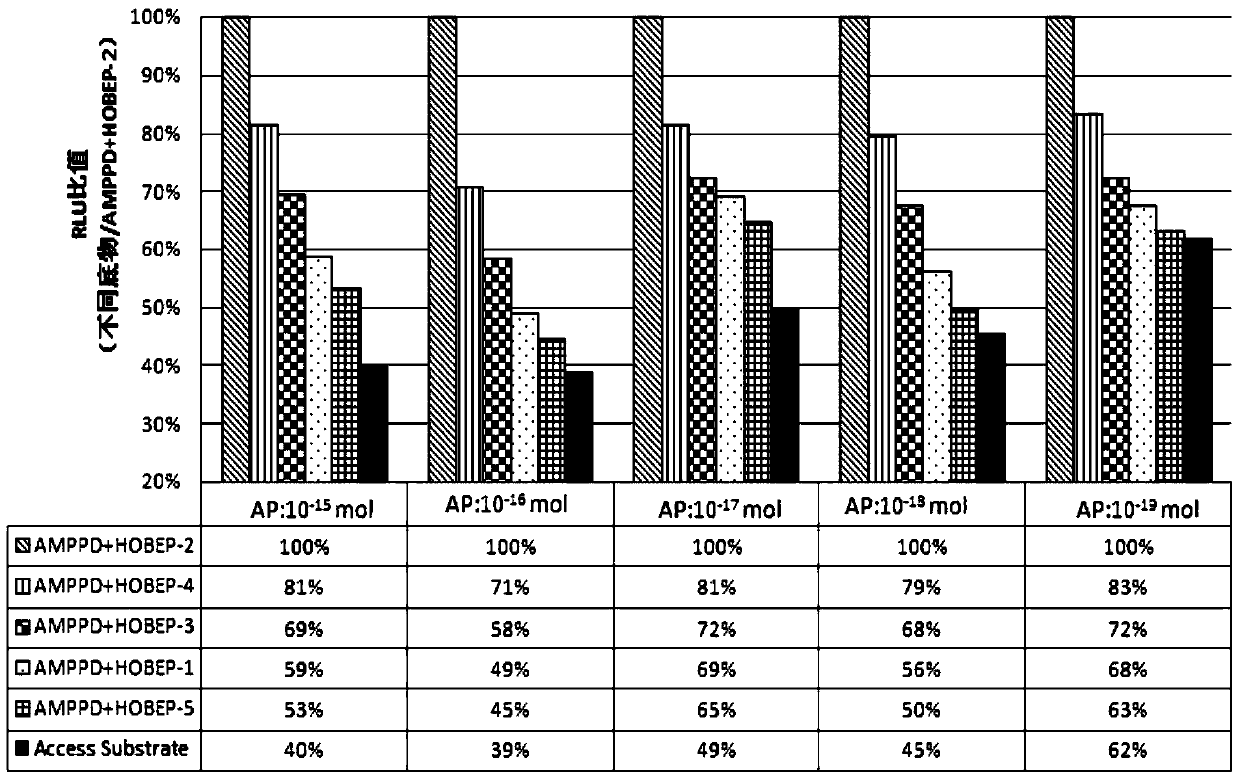
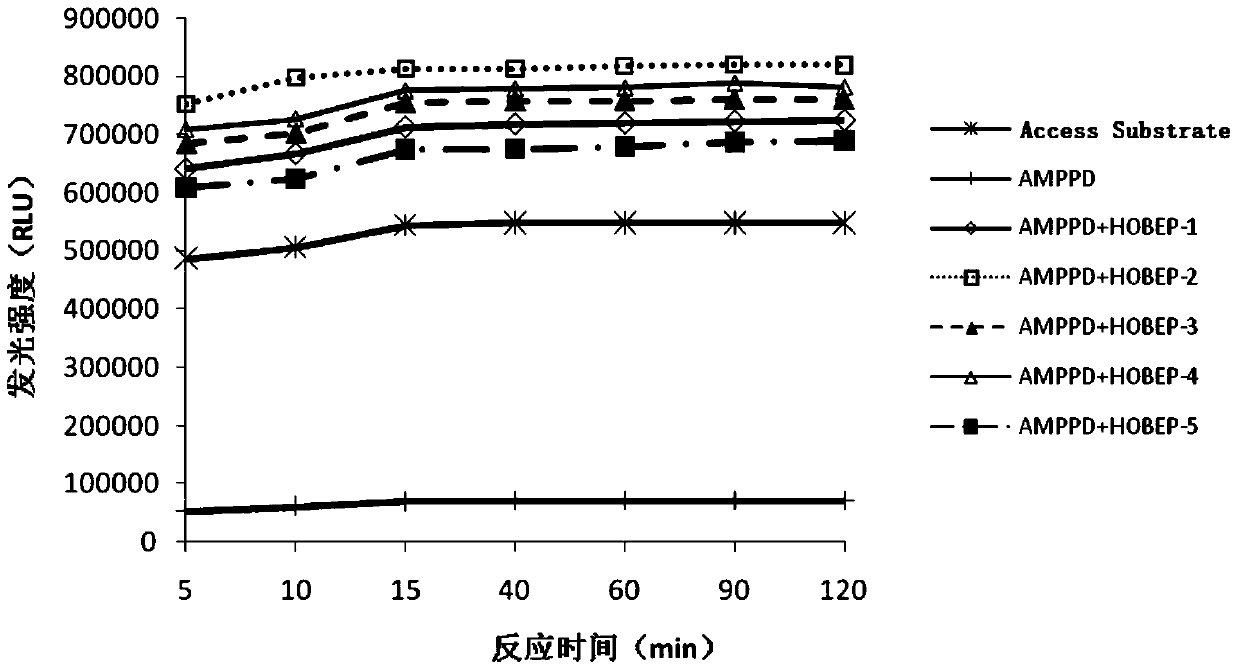
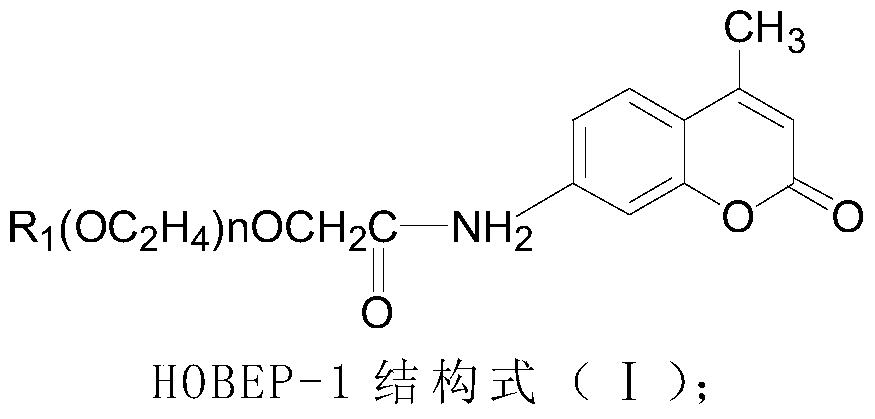
The invention relates to an enzyme-promoting chemiluminiscence substrate using alkaline phosphatase. Using water as solvent, the enzyme-promoting chemiluminiscence substrate comprises 2-amino-2-methyl-1-propyl alcohol, AMPPD, and a luminescence enhancer link-coupled by sodium fatty alcohol polyoxyethylene ether carboxylate and fluorescent compounds. According to the enzyme-promoting chemiluminiscence substrate using alkaline phosphatase, the luminescence enhancer can achieve the effect of co-surfactant, the compounds can be better combined into a chemiluminiscence buffer system, and thus the chemiluminiscence efficiency is greatly improved. The enzyme-promoting chemiluminiscence substrate using alkaline phosphatase has the advantages of being high in strength, high in sensitivity, long in duration, good in stability and the like. Clinical testing requirements can be met completely, the main performance index has already reached the level of foreign products, and the cost of the chemiluminiscence substrate is greatly lowered.
Owner:SUZHOU HAOOUBO BIOPHARML
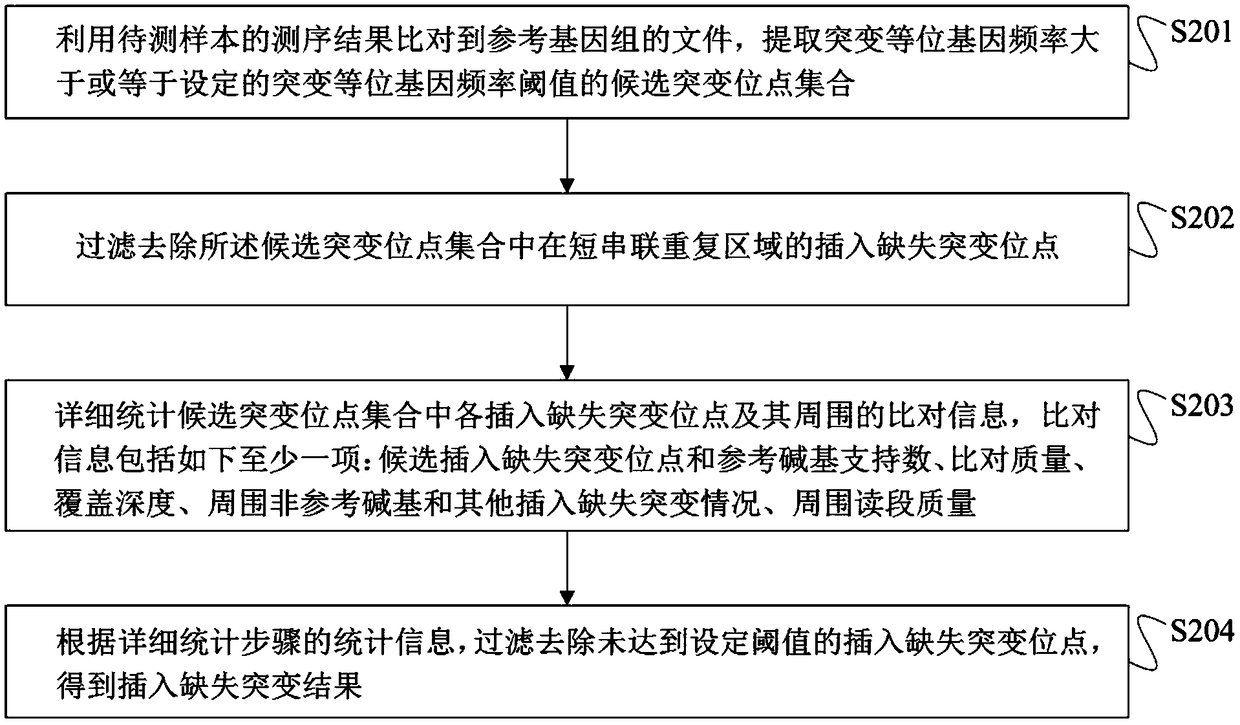
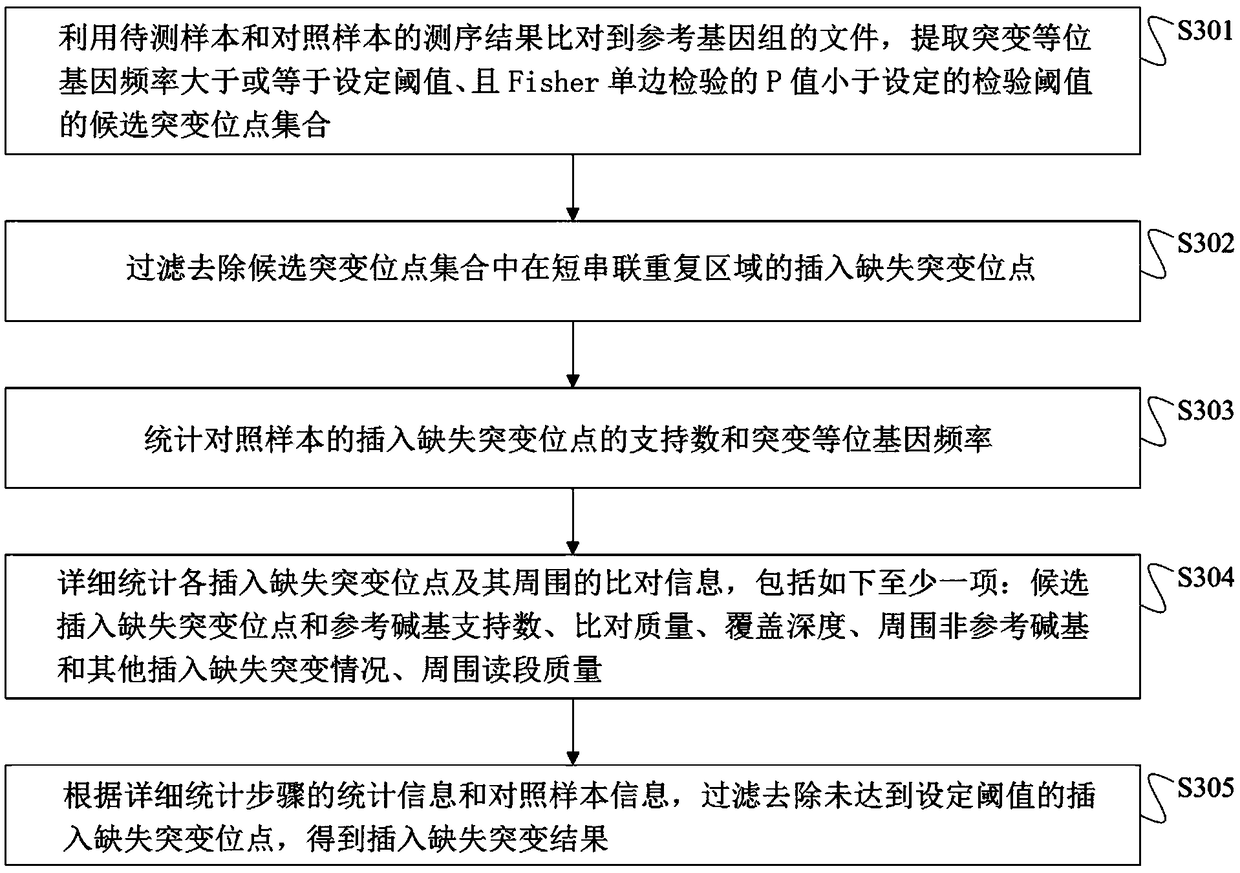
Method for detection of insertion deletion mutation based on second generation sequencing, device and storage medium
ActiveCN108690871AIncreased sensitivityStrong specificityMicrobiological testing/measurementProteomicsInsertion deletionAllele frequency
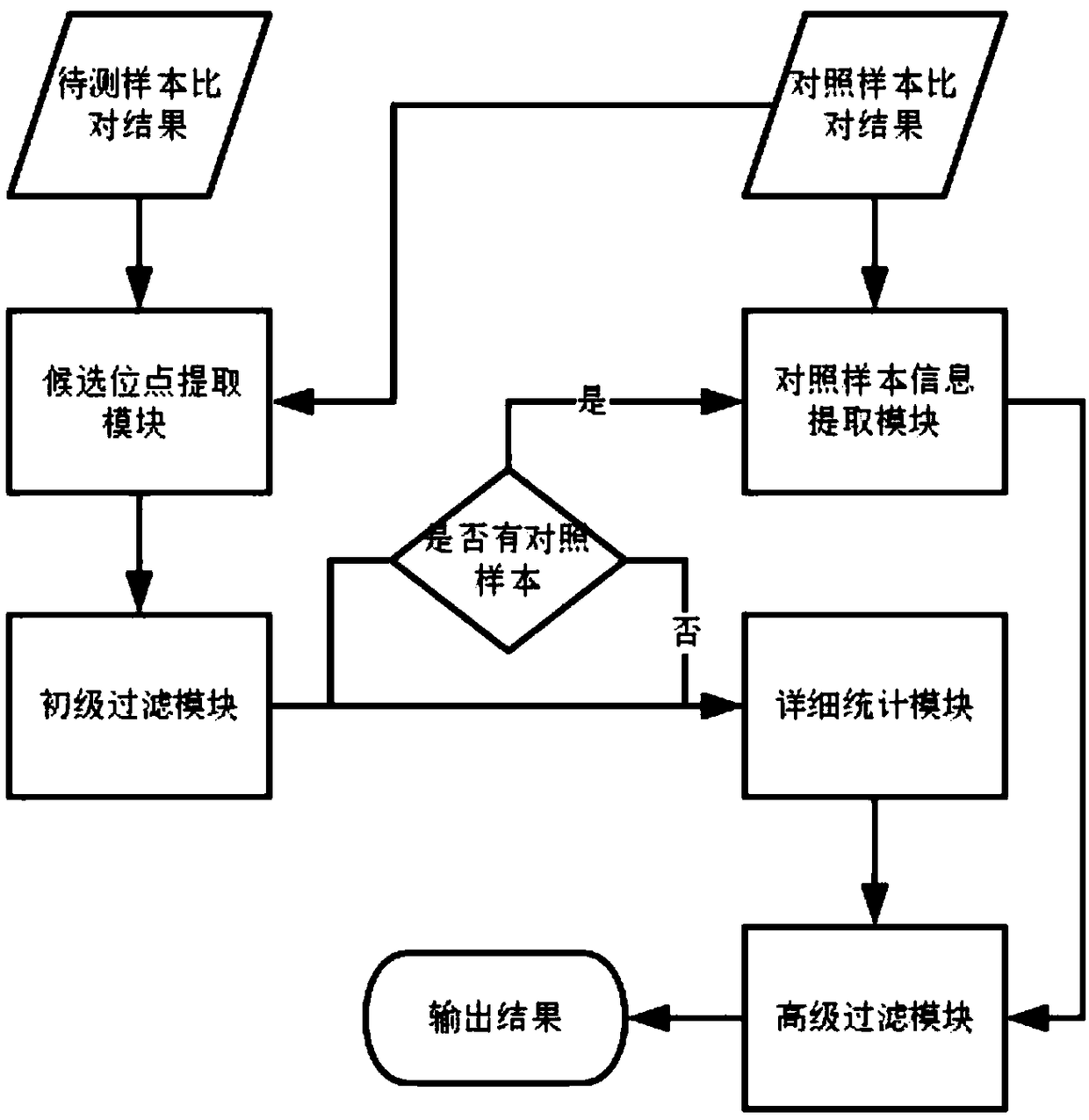
The present application discloses a method for detection of insertion deletion mutation based on second generation sequencing, a device and a storage medium. The method comprises the following steps:comparing a sample to be tested with a file of a reference genome to extract a set of candidate mutation sites with mutation allele frequency being greater than or equal to a threshold; filtering to remove sites in a short tandem repeat region; making detail statistics of comparison information of the mutation sites and comparison information surrounding the mutation sites, wherein the comparisoninformation includes InDel site and reference base support number, comparison quality, coverage depth, surrounding non-reference base and other insertion deletion mutations, surrounding read quality;and filtering to remove sites that do not reach the set threshold according to statistical information to obtain mutation results. The method does not require partial assembly, and filters second-generation sequencing data in advance to quickly eliminate most of false positive results caused by the comparison, reduces detection running time and computing resources, improves detection efficiency, has strong sensitivity and specificity, and can quickly and accurately detect InDel mutations.
Owner:深圳裕策生物科技有限公司
Detection kit for ischemia modified albumin
ActiveCN103760357AImprove binding efficiencyGuaranteed accuracyDisease diagnosisColor/spectral properties measurementsNormal albuminMedicine
The invention relates to the technical field of detection of ischemia modified albumin content, and in particular to a detection kit for ischemia modified albumin. The kit includes a reagent 1 and a reagent 2. The reagent 1 contains a buffer, cobalt chloride, a stabilizer and a preservative; and the reagent 2 contains a buffer, dithiothreitol, a stabilizer, a reducing protective agent and a preservative. The kit can accelerate the binding efficiency of cobalt ions with normal albumin, and the cobalt ions fully integrate with normal albumin in a certain period of time, so as to ensure the accuracy of the detection result on a sample by the reagent; the kit protects the stability of dithiothreitol in the solution, and does not affect the binding of dithiothreitol with cobalt ions, so as to ensure that an open reagent bottle can be stably kept in a dark place for 30 days at room temperature and a closed reagent bottle can be stably kept at 2-8 DEG C for 12 months, and fully meet the needs of clinical laboratory; and the kit accelerates the combination of dithiothreitol with cobalt ions, so that the reagents reach reaction endpoints as soon as possible, thereby ensuring the efficient detection effect of reagents and significantly improving the accuracy of reagent detection.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
High-sensitivity quantitative determination method of cardiac troponin I
ActiveCN107084966AEasy to synthesizeEasy to retouchFluorescence/phosphorescenceEnergy transferQuantitative determination
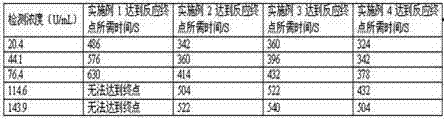
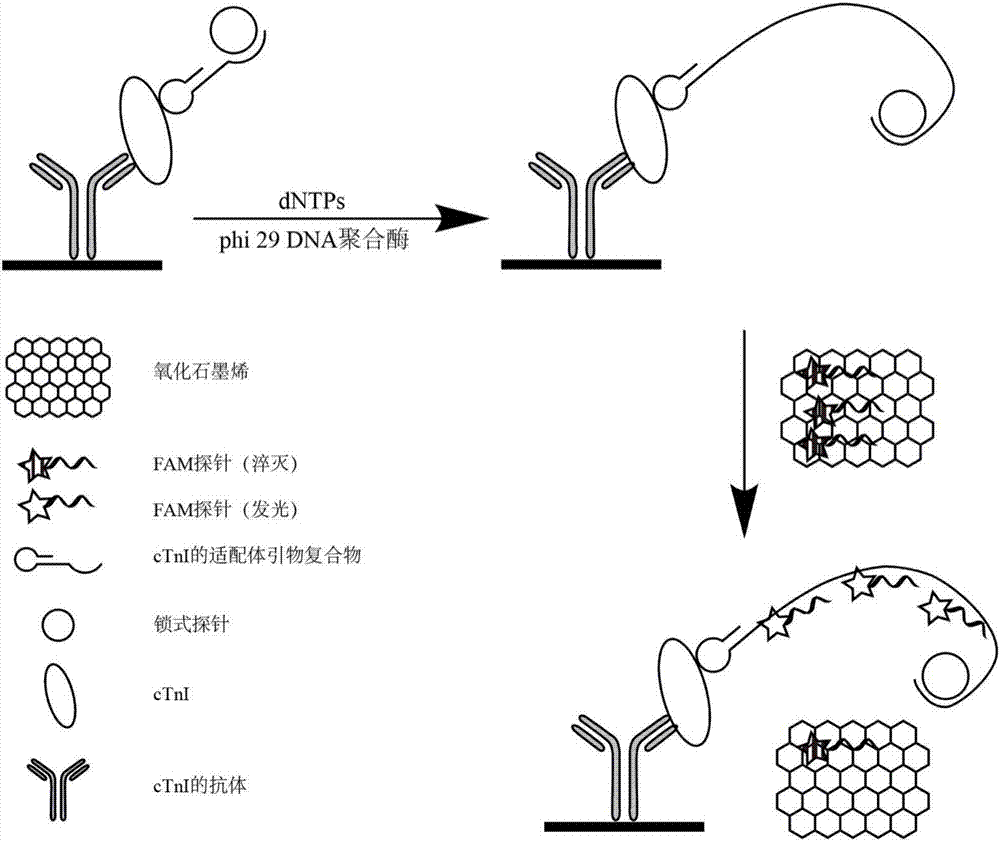
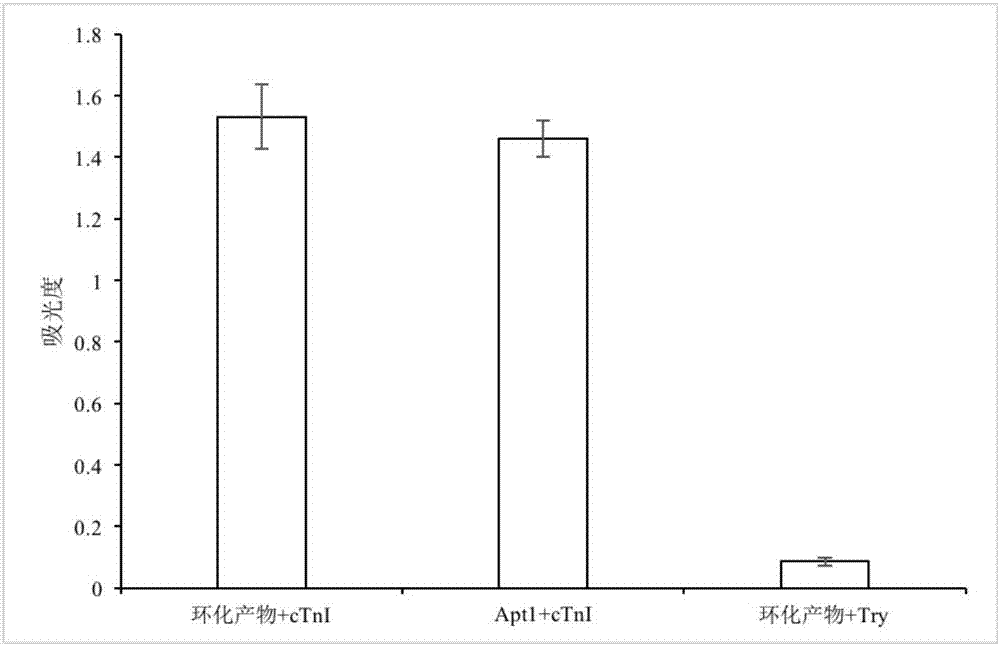
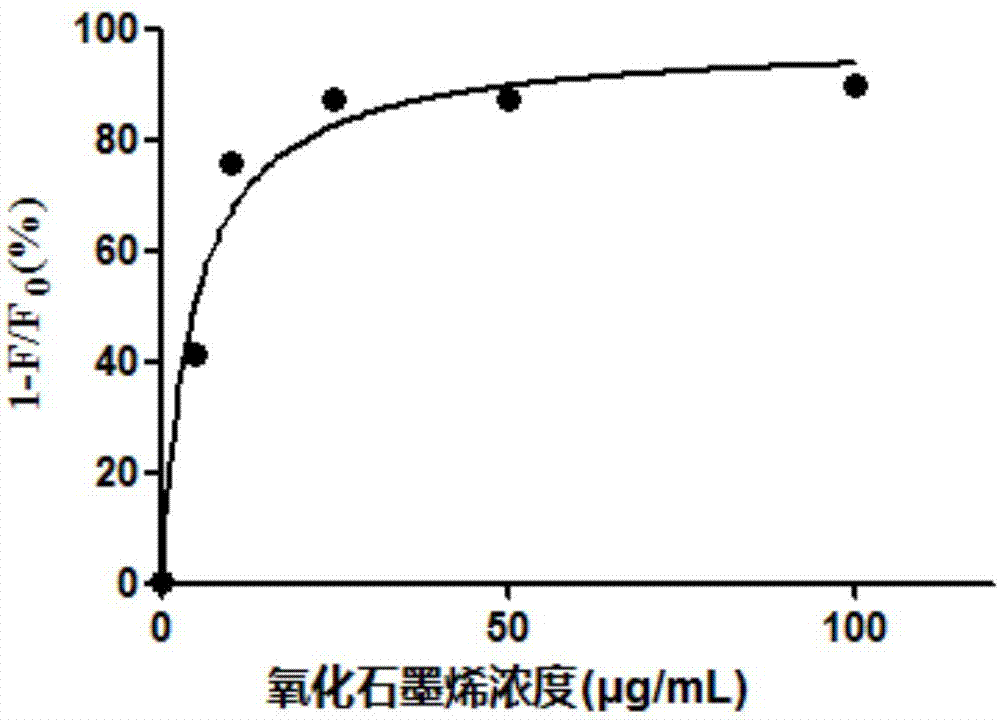
The invention relates to a high-sensitivity quantitative determination method of cardiac troponin I, and belongs to the technical field of biomedical detection. According to the detection method, specific recognition is conducted on the cardiac troponin I through an aptamer, and accurate and sensitive quantitative detection on cTnI is achieved by means of a rolling circle amplification technology of nucleic acid and a graphene oxide-based fluorescence resonance energy transfer technology; the detection method has the advantages of being good in specificity, high in sensitivity, easy and convenient to operate, low in cost and the like, and the clinical test requirements are met.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Blood cell analyzer dilution as well as hemolytic agent
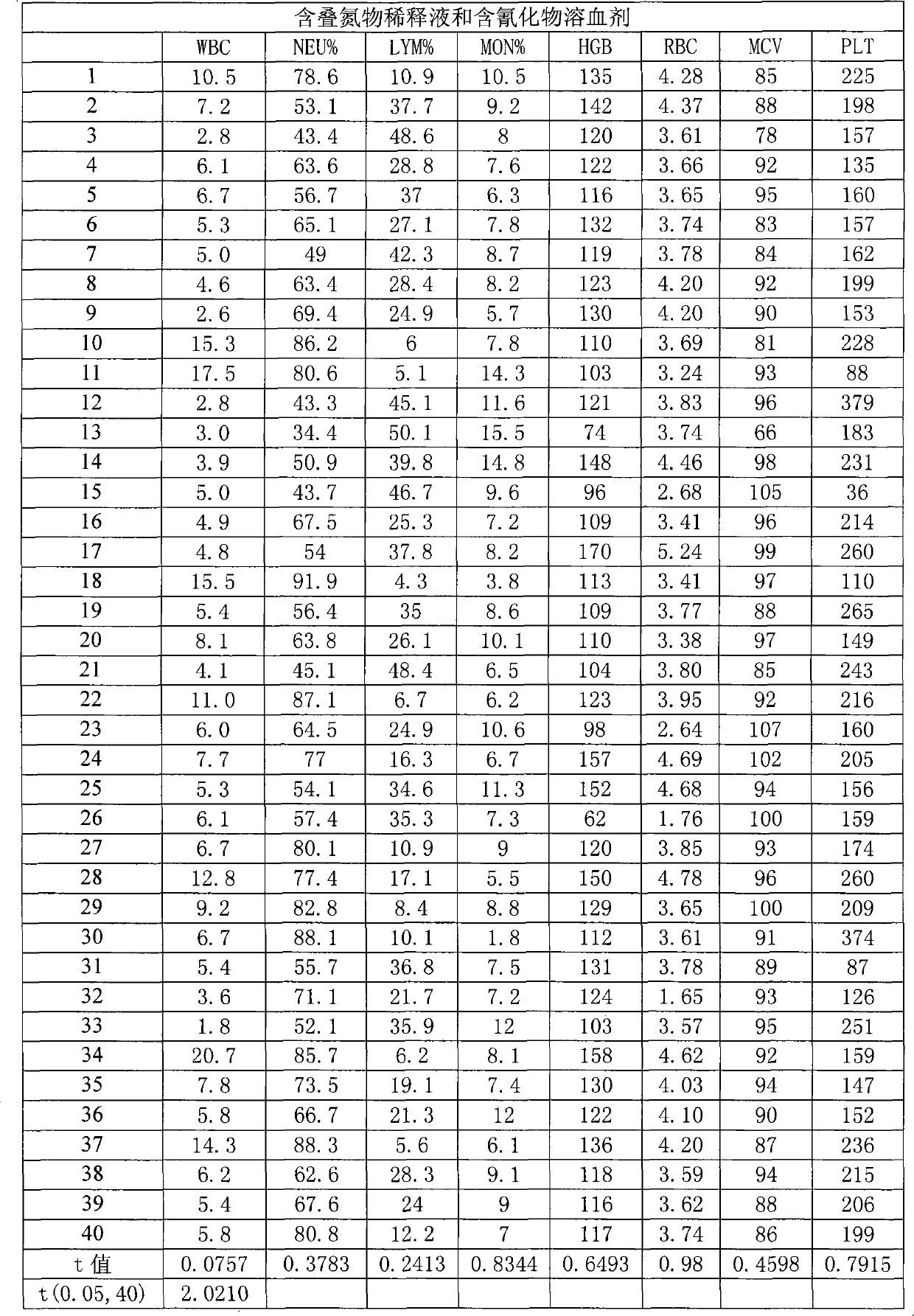
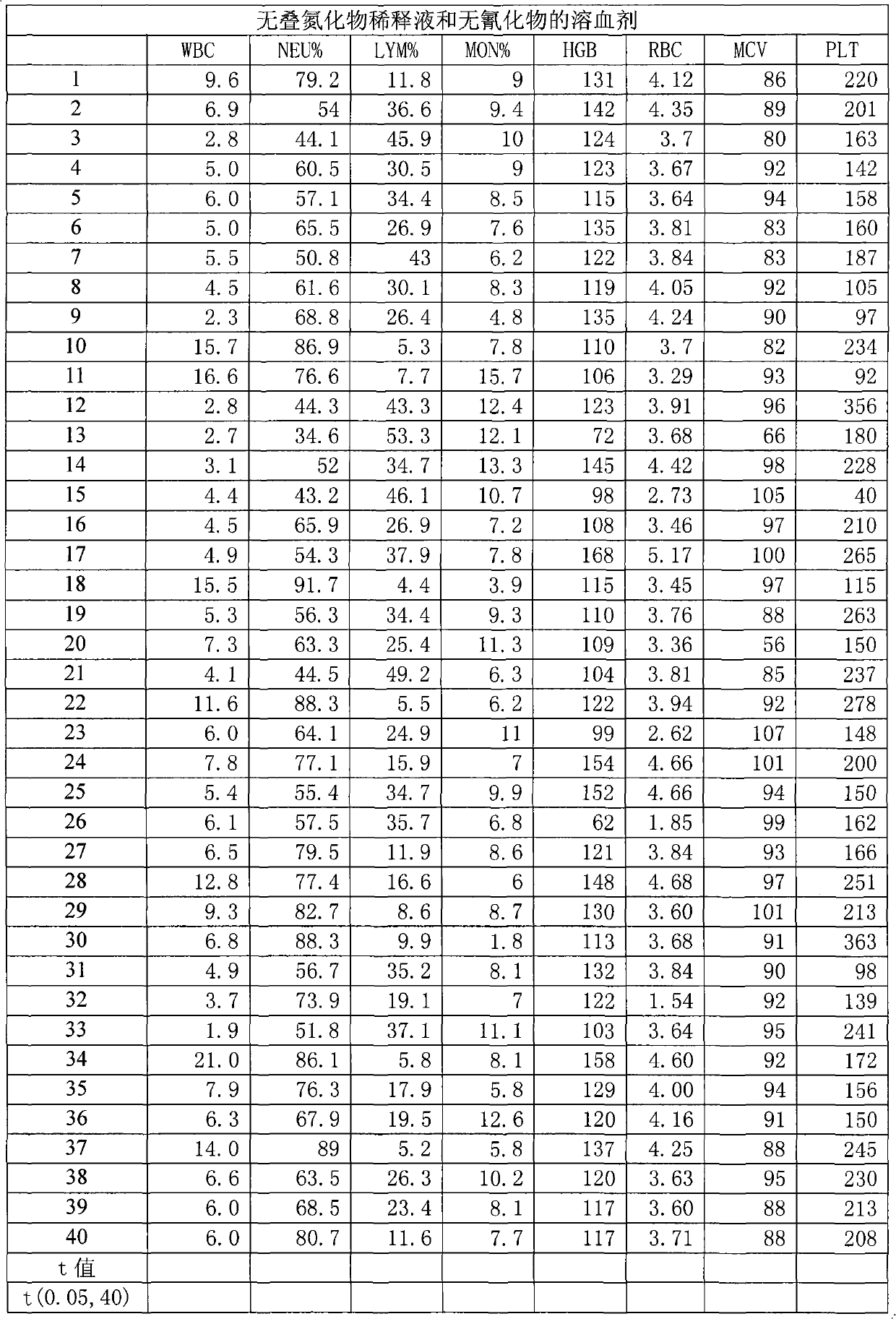
InactiveCN101281193AImprove working environmentReduce harmIndividual particle analysisBiological testingNon toxicityToxicant
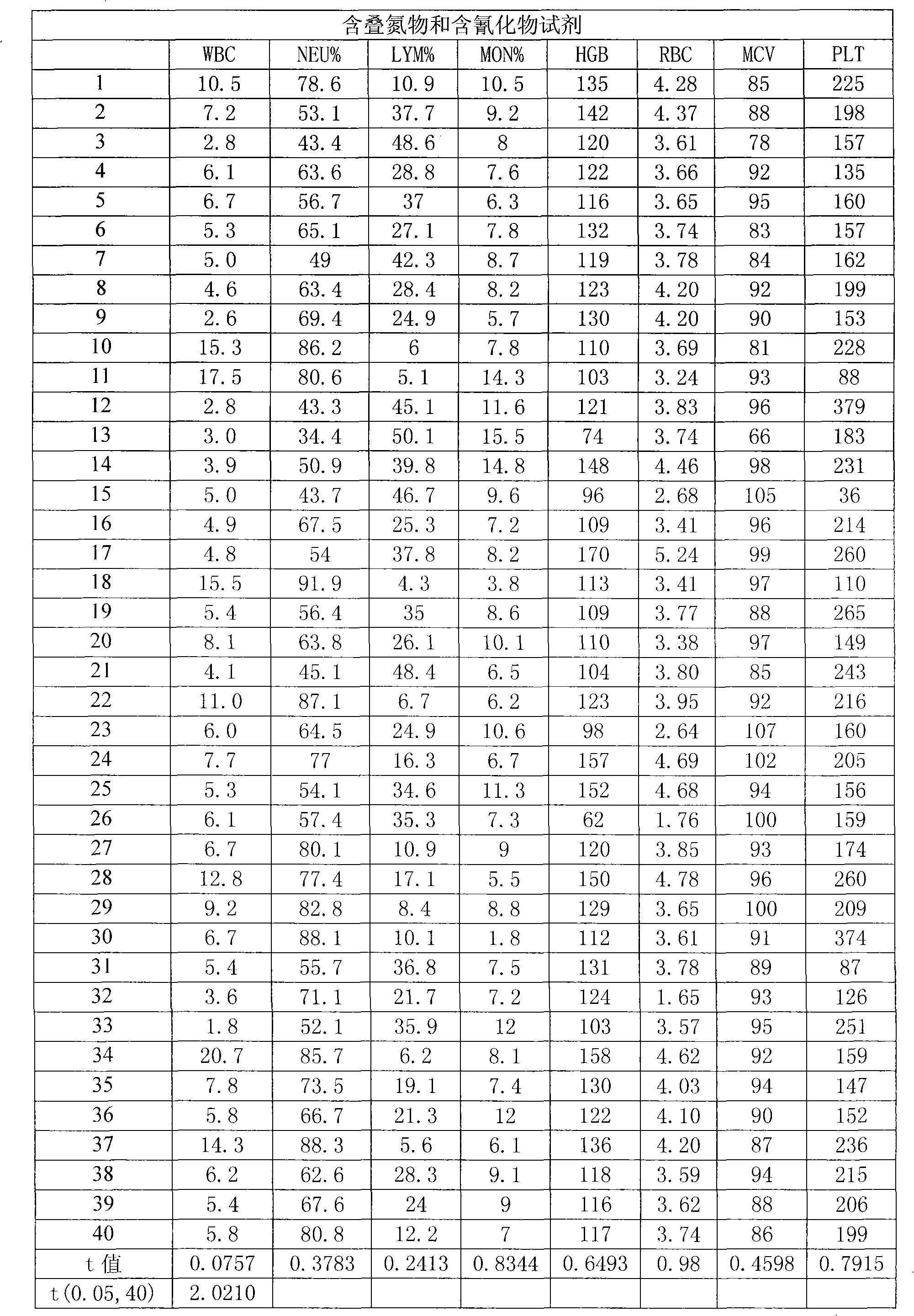
A blood cell analyzer diluent and a hemolytic agent, are characterized in that one litre diluent is provided with 12.0-4.0g of sodium chloride, 2.0-10.0g of sodium sulfate, 0.8-0.5g of 1,3,2-methylol urea, 0.2-0.5g of copper sulfite, 3.0-8.0g of EDTA-2Na, 0.2-0.7g of Piperacillin Sodium, a borate buffering liquid toning the ph value to 7.2-7.8, and the balance is water; one litre hemolytic agent is provided with 0.8-5.0g of potassium chloride, 0-60.0g of dodecyl trimethyl ammonium chloride, 14.0-0g of octadecyl trimethyl ammonium bromide, 6.0-10.0ml of isopropanol, carbonate or alcaine buffering liquid toning the ph value to 7.2-7.8, and the balance is water. The inventive reagent can form stable hemoglobin derivatives, and the absorption spectrum curves are similar when lambada is 540nm, lambada is 504nm, which can satisfy the clinical inspection requirement; the reagent does not contain cyanide, azide, and has non-toxicity, which can effective improve working atmosphere of operating staff, and can reduce harm of toxicant to personal health.
Owner:南昌百特生物高新技术股份有限公司
Glutathione reductase assay kit and preparation method and application thereof
ActiveCN108828215AImprove accuracyNo reconstitution requiredMaterial analysisPotassium ferrocyanideChemistry
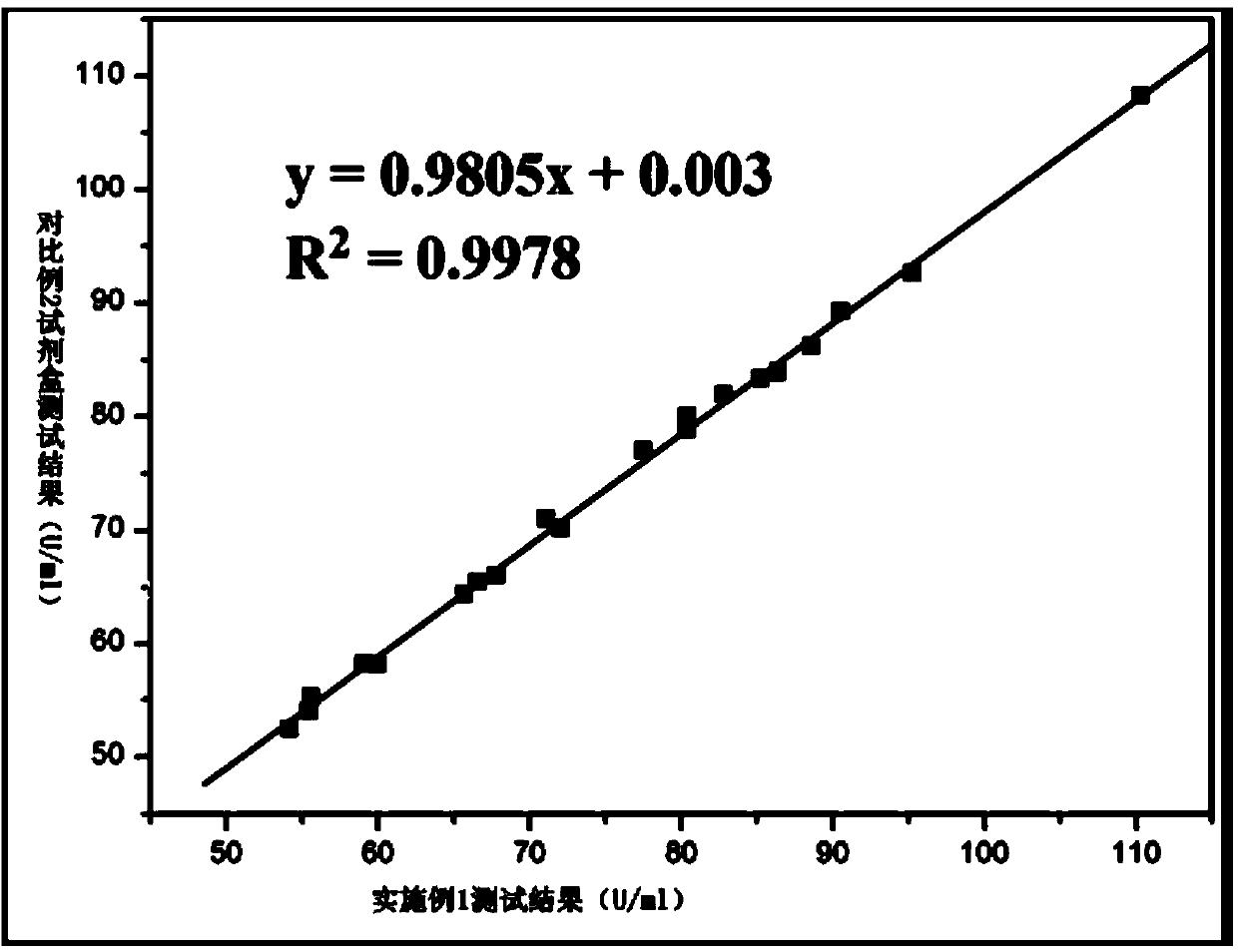
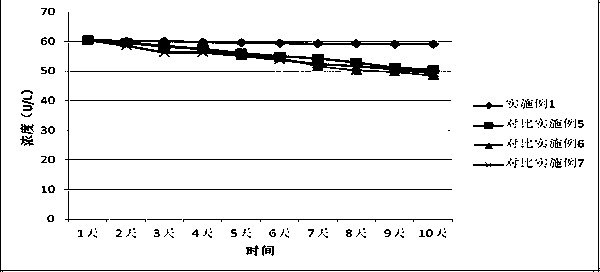
The invention provides a glutathione reductase assay kit, which comprises a reagent R1 and a reagent R2. The reagent R1 includes: 50-200 mmol / L of a Tris buffer solution, 0.5-2 mmol / L of EDTA, 1-5 kU / L of pyruvic carboxylase, 1-5 kU / L of ascorbic acid oxidase, 5-20 mg / L of potassium ferrocyanide, a surfactant and a preservative; the reagent R2 includes: 50-200 mmol / L of the Tris buffer solution, 0.5-2 mmol / L of EDTA, 2-10 mmol / L of GSSG, 0.1-0.5 mmol / L of NADPH, 1-15 g / L of a stabilizer, and 0.5-2 g / L of a preservative. The liquid assay kit has good stability and is strong in anti-interferenceeffect. The invention also discloses a preparation method and an application of the assay kit.
Owner:中拓生物有限公司
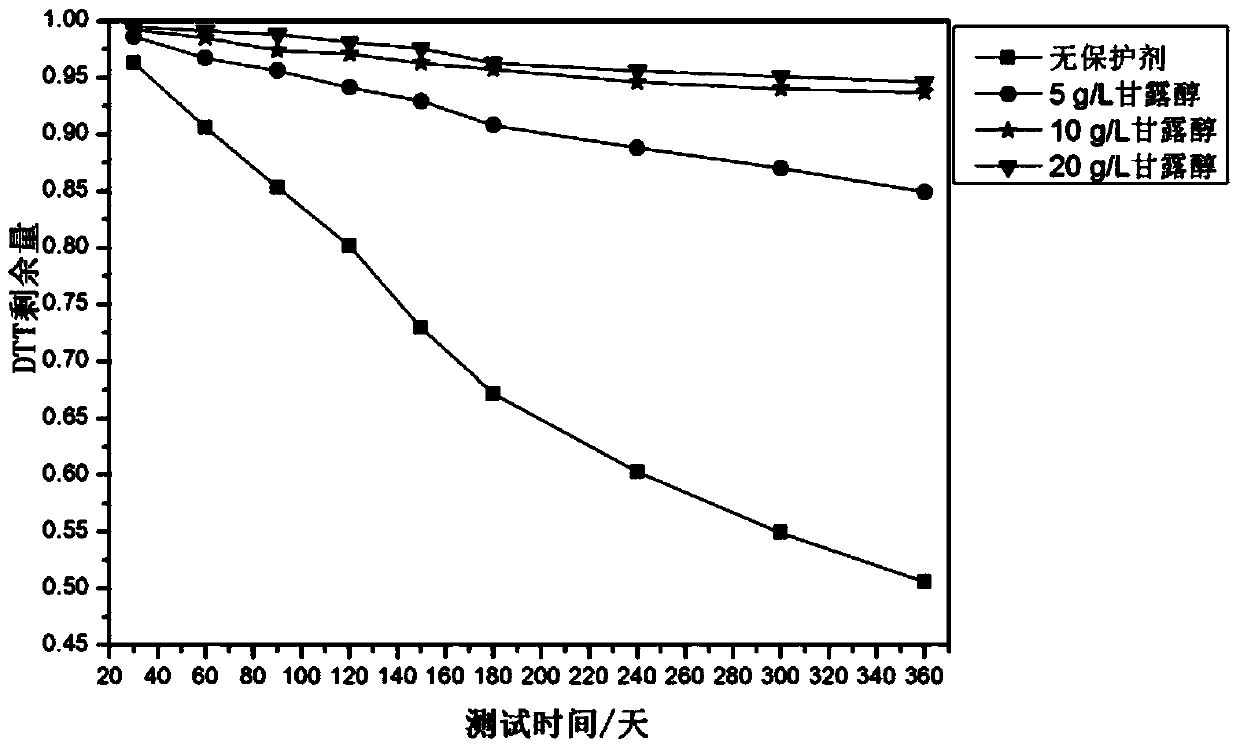
Stable ischemia modified albumin testing kit
ActiveCN102147416AImprove stabilityReduce the binding forceColor/spectral properties measurementsBiological testingRoom temperatureOxygen
The invention discloses a stable ischemia modified albumin testing kit which comprises a cobalt ion reagent and a dithiothreitol (DTT) reagent. A small amount of nonionic surfactant is added in the dithiothreitol reagent, thus the combination of DTT in the reagent and free oxygen is greatly reduced, the oxidation speed of DTT is reduced, and the stability of the reagent is remarkably improved. Simultaneously, the concentration of DTT is increased, thus the stability of the kit is improved to a certain extent. The stable ischemia modified albumin testing kit disclosed by the invention can be stored stably in an opened bottle in a dark place at the room temperature for 30 days, and also can be stored stably in a closed bottle at the temperature of 2 to 8 DEG C for 12 months, and can totally meet the requirements for clinical examinations.
Owner:SICHUAN XINCHENG BIOLOGICAL CO LTD
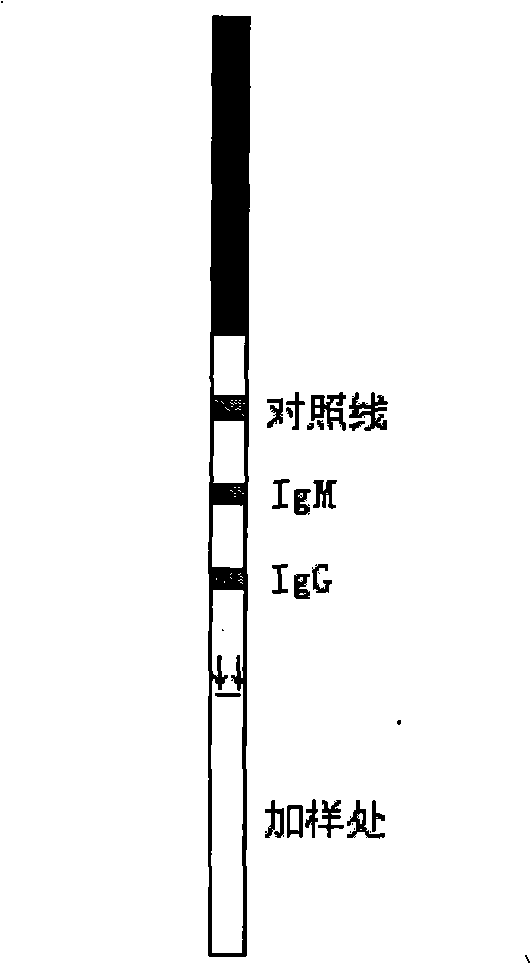
Gold-labeled test paper strip for quick diagnosis of hemorrhagic fever with renal syndrome
InactiveCN1800854AHigh sensitivityReduce fatality rateSugar derivativesPreparing sample for investigationAntigenDiagnosis methods
The invention discloses a recombination antigen for HFRS diagnosis and its rapid GIA diagnosis indicator paper opposite to shortened NP recombination antigen e112 of Hantaan virus by gene clone technique and simultaneous-detected IgM and IgG antigens in patient serum. The said test paper overcomes the limitation of current HFRS diagnosis method, benefit to early-stage diagnosis, reduces illness death rate, does not requires special device nor professional staff to meet more detection need, and cuts cost greatly.
Owner:FUJIAN CENT FOR DISEASE CONTROL & PREVENTION
Steady ischemia modified albumin kit
ActiveCN104198729AImprove test performanceImprove stabilityMaterial analysis by observing effect on chemical indicatorDisease diagnosisSurface-active agentsIschemia-modified albumin
The invention relates to a steady ischemia modified albumin kit, which comprises a cobalt ion reagent and a dithiothreitol reagent, wherein the cobalt ion reagent comprises the following components: 50-500 mmol / L of buffer solution, 0.01-1.0 g / L of cobalt chloride, 1 g / L of surface active agent, and 0.5 g / L of preservative; and the dithiothreitol reagent comprises the following components: 50-300 mmol / L of buffer solution, 0.01-1.0 g / L of dithiothreitol (DTT), 1 g / L of surface active agent, 1-20 g / L of protective agent, and 0.5 g / L of preservative. The kit disclosed by the invention can reach reaction endpoint rapidly and is steady in property; after being opened, the kit can be stably stored in a dark place at room temperature for 30 days; after being closed, the kit can be stably stored at 2 DEG C -8 DEG C for 12 months, and therefore, clinical test requirements can be completely satisfied.
Owner:NINGBO RUI BIO TECH
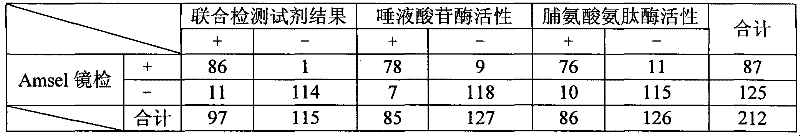
Joint detection reagent for bacterial vaginosis
InactiveCN101748188AAvoid missing detectionHigh sensitivityMicrobiological testing/measurementMicroorganism based processesBacterial vaginosisP-Nitroaniline
The invention relates to a joint detection reagent for bacterial vaginosis, which comprises a sialidase activity detection reagent and a praline aminopeptidase activity detection reagent, wherein the sialidase activity detection reagent consists of the following substances: 0.5-2.5g / l enzyme substrate A, 30-60g / l carbohydrate and 1-2g / l citric acid; the enzyme substrate A is 5-bromine-4-chlorine-3-indol-N-acetyl-alpha-sialic acid glycosides, nitrobenzene-N-acetyl-alpha-sialic acid glycosides or naphthol-N-acetyl-alpha-sialic acid glycosides; the praline aminopeptidase activity detection reagent consists of the following substances: 2-5g / l enzyme substrate B, 0.1-0.3mol / l Tris-hydrochloric acid buffer solution and 10-20g / l N-glycylglycine; and the enzyme substrate B is L-proline-paranitroaniline, L-proline-alpha-naphthylamine or L-proline-beta-naphthylamine. The reagent is used conveniently, special devices such as a microscope and the like are not required in detection, the detection speed is high, the sensitivity and the specificity are high, BV detection miss caused by merely detecting the activity of one enzyme is effectively avoided, and the reagent is popularized easily.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Blood corpuscle detection and biochemical detection instrument and detection method thereof
InactiveCN106370584AAvoid repetitionShorten detection timeScattering properties measurementsBiological particle analysisHemolytic AgentsMedicine
The invention discloses a blood corpuscle detection and biochemical detection instrument and a detection method thereof. The detection instrument comprises a transfer module, a dilution liquid addition module, a hemolytic agent addition module, a cleaning agent addition module, a first detection cup, a second detection cup, a cleaning module, a uniform mixing module and a control module. The first detection cup is used for first dilution of a blood sample, simultaneous detection of leucocyte and ferrohemoglobin indexes in the same blood sample and detection of the other one biochemical component in the blood sample. The second detection cup is used for second dilution of a blood sample and detection of erythrocyte and platelet in the dilute blood sample. The detection instrument realizes fast blood routine detection and other biochemical component detection through the same biochemical detection instrument, has complete functions, realizes fast clinical urgent blood routine detection and biochemical index detection, has low sample consumption, has simple operation processes and produces few wastes.
Owner:SINNOWA MEDICAL SCI & TECH
Preserving fluid as well as preparation method and application thereof
The invention relates to the technical field of biomedicines, in particular to a preserving fluid as well as a preparation method and application thereof. The invention discloses the preserving fluidwhich comprises the following raw materials: a platelet aggregation inhibitor, a cell membrane protector, a permeability protector, a pH regulator and an anticoagulation agent. The preserving fluid iscapable of maintaining activity of circulating tumor cells and reducing erythrocyte or platelet aggregation.
Owner:GUANGZHOU IMPROVE MEDICAL TECH CO LTD
Long-optical-path enzyme-linked immunoassay for testing thyroid stimulating hormone, and kit
ActiveCN102192888AHigh sensitivityLarge specific surface areaColor/spectral properties measurementsBiological testingOptical pathlengthEnzyme linked immunoassay
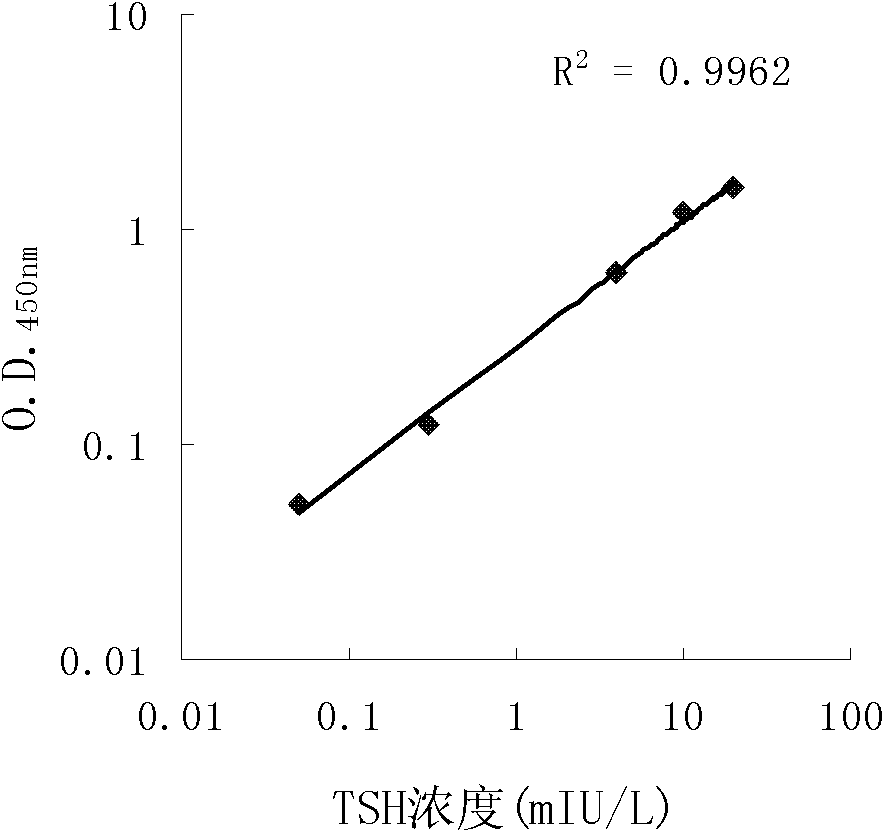
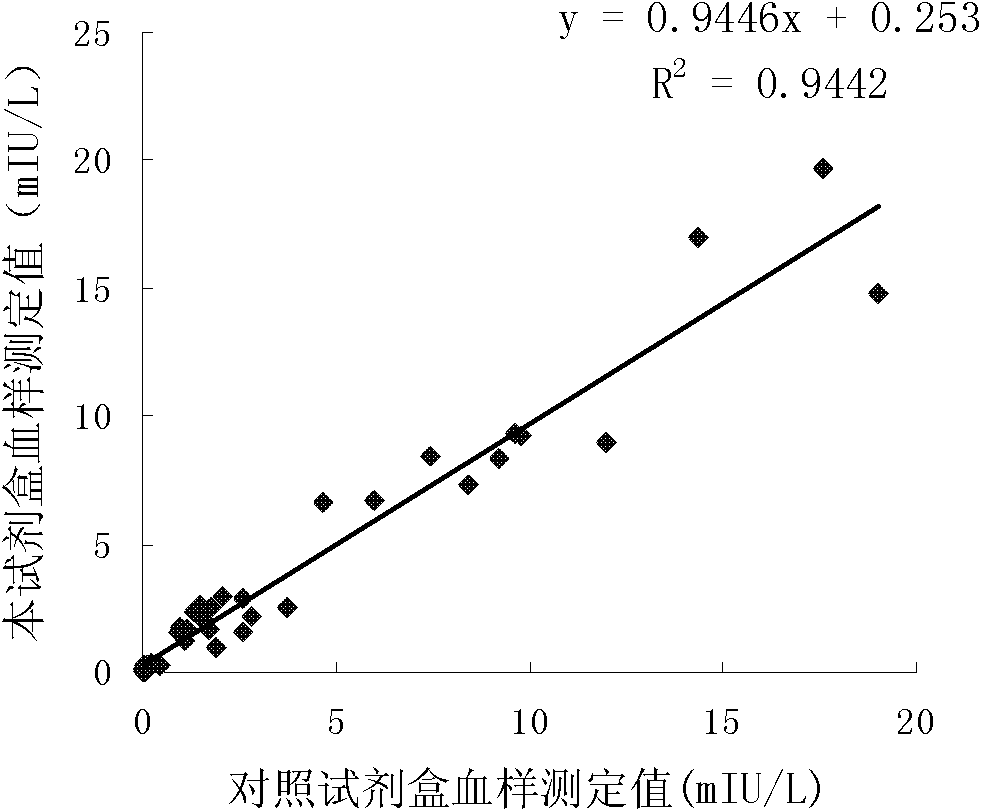
The invention discloses long-optical-path enzyme-linked immunoassay for testing thyroid stimulating hormone and provides a kit of the method. The long-optical-path enzyme-linked immunoassay comprises the following test steps of: performing immune reaction, separating, developing, measuring and processing data. A long-optical-path flowing sample pool and an optical fiber spectrometer are adopted for measurement; the detection sensitivity is 0.015mIU / L by increasing a measuring optical path, adopting a low-background substrate solution as well as optimizing a component preparation method; and compared with the sensitivity of the conventional optimized enzyme-linked immunoassay in which the same antibody is used, the sensitivity is enhanced by four times. The test linear range is 0.05 to 20mIU / L. The immune reaction is performed in a wrapped tube, and the reaction mode is a dual antibody sandwich one-step method. The kit comprises an antibody coated tube, a serial calibrator, an enzyme marker, a condensed cleaning solution, the low-background substrate solution, a terminating solution and an end-product diluent. The method is high in sensitivity and proper in test range, and the requirement of clinical detection can be met; meanwhile, the kit has low manufacturing cost and is favorable to popularization and application.
Owner:HTA CO LTD
Rapid 1-type dengue gold-marking diagnosis test paper strip
InactiveCN102778565AEasy accessPrevent proliferationVirus peptidesMicroorganism based processesAntigenImmunologic function
The invention discloses an recombinant antigen and a rapid gold-marking diagnosis paper strip for 1-type dengue. The invention mainly aims to provide an outer-membrane protein DIII region recombination antigen for the 1-type dengue diagnosis and can simultaneously aims to a rapid gold-marking diagnosis test paper strip used for detecting IgM, IgG antibodies in sera of 1-type dengue virus infected persons. The test paper strip utilizes a gene clone technology to obtain the outer-membrane protein DIII region recombination antigen of a 1-type dengue virus, and the outer-membrane protein DIII region recombination antigen can be used for immunology diagnosis after purification; the rapid gold-marking diagnosis test paper strip can simultaneously be used for detecting the IgM, IgG antibodies in the sera of the 1-type denguen virus infected persons, so as to judge the infection states of patients, to do early diagnosis on the patients, so that the important action for controlling an infection source and reducing disease spread; in addition, according to the gold-marking diagnosis test paper strip, a specific instrument is not required, and well-trained professional staff is not required, so that requirements of clinic detection in substrate hospitals and rural health centers can be met; and the cost is greatly reduced, the convenience is brought to the patients, and the test paper strip is suitable for large-scale seroepidemiological survey.
Owner:严延生
Glutathione peroxidase determination kit, preparation method of glutathione peroxidase determination kit and application of glutathione peroxidase determination kit
The invention discloses a glutathione peroxidase determination kit, the kit contains a reagent R1 and a reagent R2; the reagent R1 contains buffer solution, EDTA, glutathione reductase, reduced glutathione, potassium chloride, magnesium chloride, surfactant, stabilizer and preservative; and the reagent R2 contains the buffer solution, the EDTA, NADPH, cumene hydrogen peroxide, surfactant, stabilizer and preservative. The invention also discloses a preparation method of the glutathione peroxidase determination kit and application of the glutathione peroxidase determination kit. The kit providedby the invention is a high-sensitivity and excellently stable liquid kit.
Owner:中拓生物有限公司
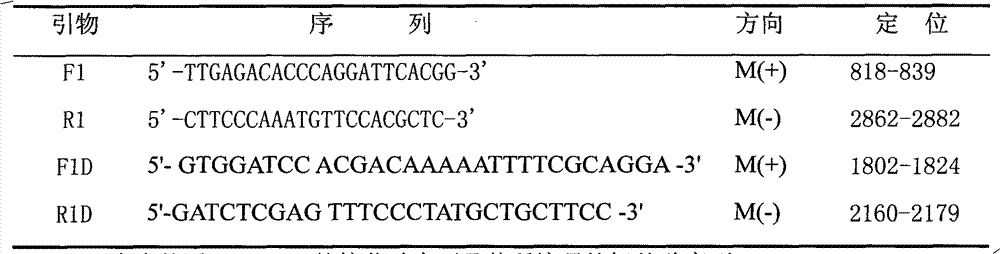
Porcine reproductive and respiratory syndrome and porcine Japanese B encephalitis dual one-step RT-PCR (Reverse Transcription-Polymerase Chain Reaction) diagnosis kit
ActiveCN103215389AGood sensitivity and specificityEasy to operateMicrobiological testing/measurementMicroorganism based processesMolecular biologyJapanese encephalitis viruses
The invention discloses a porcine reproductive and respiratory syndrome and porcine Japanese B encephalitis dual one-step RT-PCR (Reverse Transcription-Polymerase Chain Reaction) diagnosis kit which comprises 100 micro-milliliters of DL2000, 20 micro-milliliters of RT=PCR one-step enzyme, 250 micro-milliliters of enzyme buffer solutions, 300 micro-milliliters of RNase Free dH2O, 80 micro-milliliters of mixed primers ( respectively with the concentration of 10 micromoles / liter), 20 micro-milliliters of positive controls and 20 micro-milliliters of negative controls. According to the invention, two pairs of primers are designed according to the sequences of a PRRSV (Porcine Reproductive and Respiratory Syndrome Virus) OFR7 N gene and a JEV (Japanese Encephalitis Virus) NS1 gene which are contained in a GenBank; and the dual one-step RT-PCR diagnosis kit for detecting a porcine reproductive and respiratory syndrome virus and a porcine Japanese B encephalitis virus is successfully developed through the optimization of reaction conditions, and the dual one-step RT-PCR diagnosis kit has sensibility and specificity on the two viruses. The dual one-step RT-PCR diagnosis kit disclosed by the invention can be used for clinically detecting the porcine reproductive and respiratory syndrome and the porcine Japanese B encephalitis.
Owner:GUIZHOU INST OF ANIMAL HUSBANDRY & VETERINARY
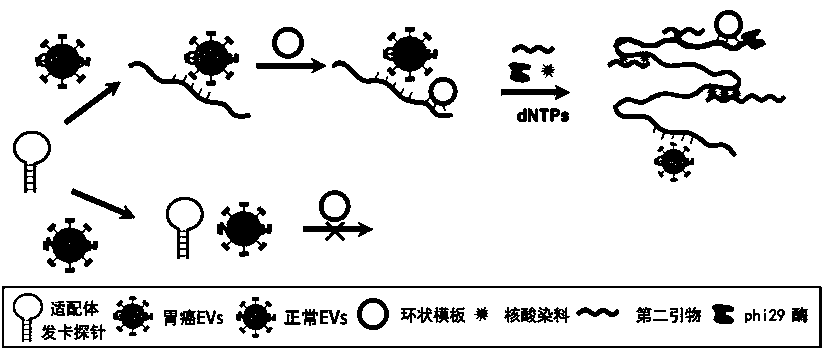
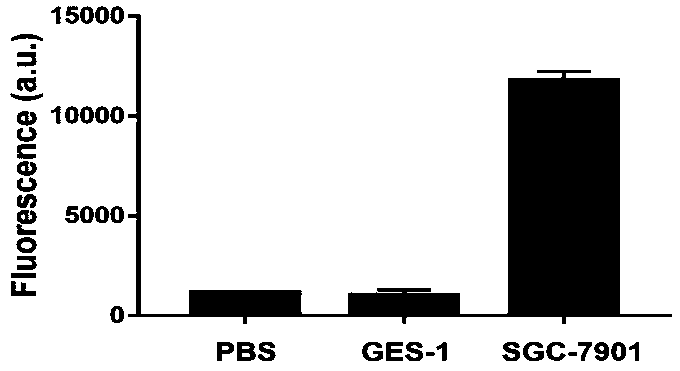
Extracellular vesicle detection technology based on aptamer hairpin-type triggered super-branch rolling ring amplification
PendingCN111394433AEasy to operateAvoid extracellular vesicle lossMicrobiological testing/measurementMolecular biologyBiophysics
The invention discloses an extracellular vesicle detection technology based on aptamer hairpin-type triggered super-branch rolling ring amplification. An aptamer hairpin part is firstly designed by taking an extracellular vesicle surface protein as a target, and a corresponding padlock probe, a connecting sequence and a second primer are designed; an annular template is prepared by hybridization of the padlock probe and the connection sequence; after the aptamer hairpin part is incubated with extracellular vesicles, a hairpin structure is opened so as to be combined with the annular template,the super-branch rolling ring amplification is triggered under the condition that the second primer is added, and a large amount of length gradient double-stranded nucleic acid is formed; a strong fluorescence signal can be generated in combination with a nucleic acid dye SYBR Green I, and the intensity of the fluorescent signal and the extracellular vesicle concentration are positively correlated, so that the target extracellular vesicles can be quantitatively detected according to the fluorescence intensity, and the free-state aptamer hairpin part does not need to be cleaned based on the fact that the free-state aptamer hairpin part is not able to trigger amplification. The extracellular vesicle detection technology provided by the invention is simple and high in specificity, the extracellular vesicles are free of cleaning, and high sensitivity is achieved.
Owner:NANJING DRUM TOWER HOSPITAL
LAMP detection method for babesia bovis
ActiveCN101974621AQuick checkAccurate detectionMicrobiological testing/measurementFluorescence/phosphorescencePositive controlTherapeutic effect
The invention provides a loop-mediated isothermal amplification (LAMP) detection method for babesia bovis. The method comprises the following steps of: extracting deoxyribose nucleic acid (DNA) of a specimen to be detected; setting an LAMP detection kit; performing LAMP amplification; analyzing amplification products; and determining by comparing color change of the specimen to be detected, a positive control and a negative control. The method can conveniently, quickly and accurately detect the babesia bovis in the specimen to be detected, and can be used for surveying molecular epidemiology of the babesia bovis and monitoring treatment effects. By the detection method, a template is easy to prepare, the cost is low, and the specificity and sensitivity can be improved. The result can be observed by naked eyes by adding an appropriate amount of SYBR GREEN I dyes, the instrument requirement is low, the consumed time is short, the result judgment is simple, the specificity and the sensitivity are high, the requirements of clinical detection can be met, and the prospect is wide.
Owner:ZHEJIANG UNIV
Trace detection method of vitamin A and vitamin E in peripheral blood
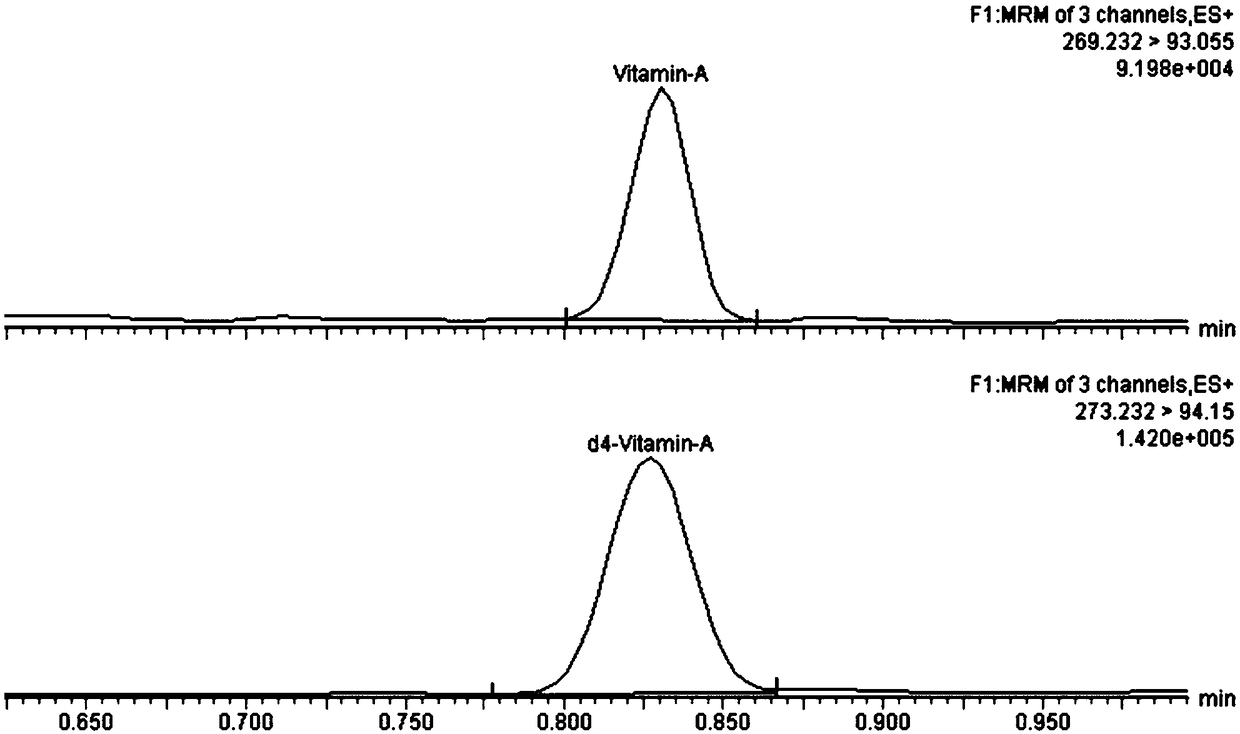
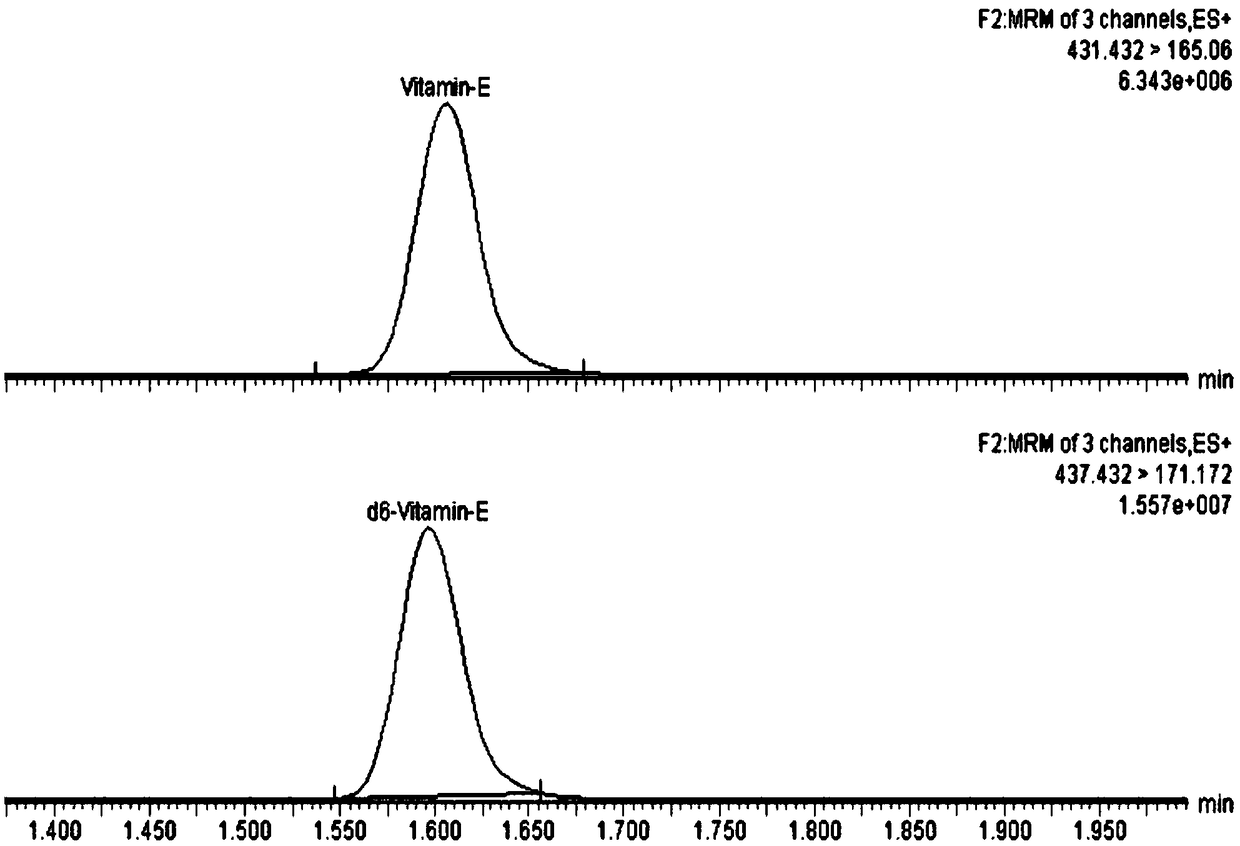
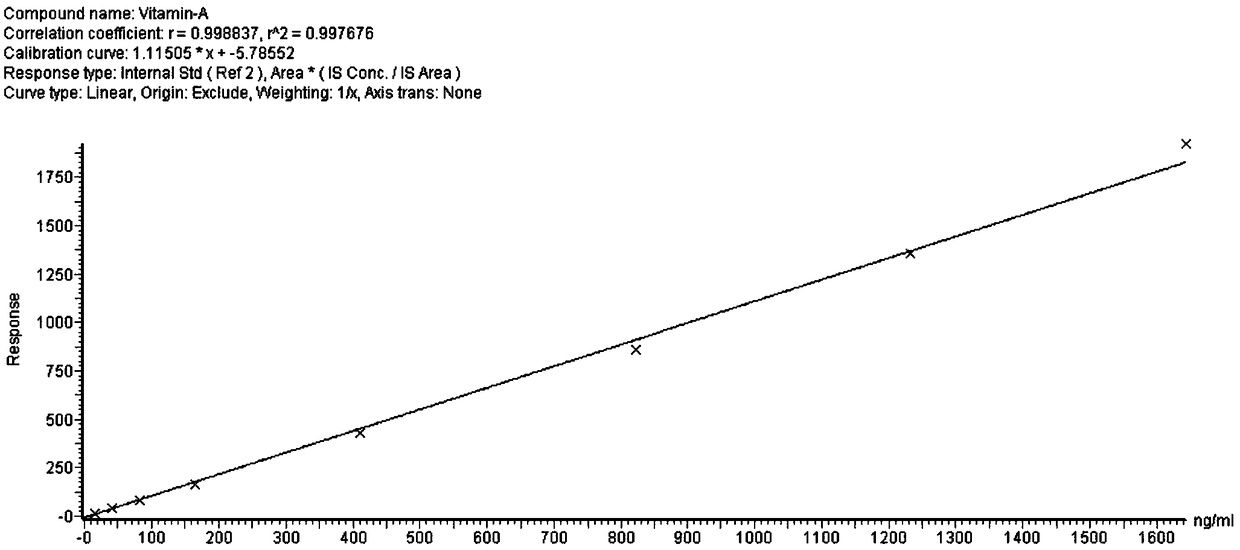
InactiveCN108802221AThe pretreatment steps are simple, convenient and effectiveImprove efficiencyComponent separationDissolutionPeak area
The invention discloses a trace detection method of vitamin A and vitamin E in peripheral blood. The trace detection method is characterized by comprising the following steps: carrying out proprocessing on to-be-detected samples; taking peripheral blood samples and adding solutions containing internal standard; carrying out vortex oscillation to complete protein precipitation; adding tertiary butyl ether and carrying out vortex oscillation and extraction; centrifuging supernatant; blow-drying the supernatant by using nitrogen at a room temperature; adding a re-dissolution liquid; carrying outvortex oscillation and centrifugation to obtain the to-be-detected samples; carrying out ultrahigh performance liquid chromatography tandem mass spectrometry detection; obtaining the peak area of to-be-detected matter and the peak area of the corresponding internal standard in the to-be-detected samples through the ultrahigh performance liquid chromatography tandem mass spectrometry. According tothe trace detection method provided by the invention, high-speed high-efficiency detection on the vitamin A and the vitamin E in the trace peripheral blood samples can be carried out in 2 minutes.
Owner:杭州度安医学检验实验室有限公司
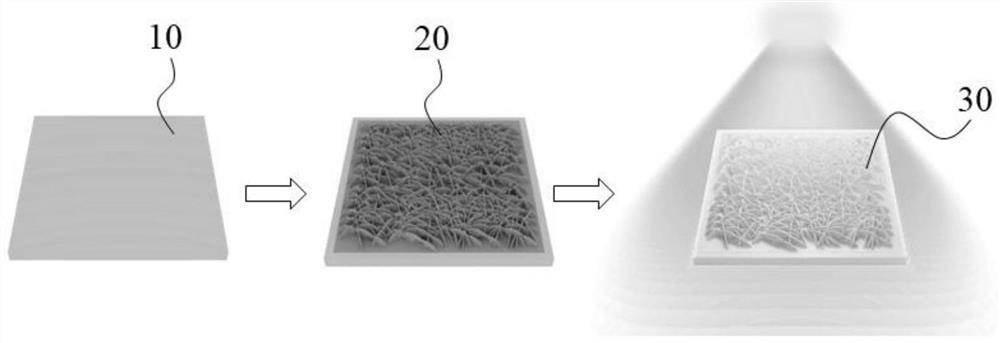
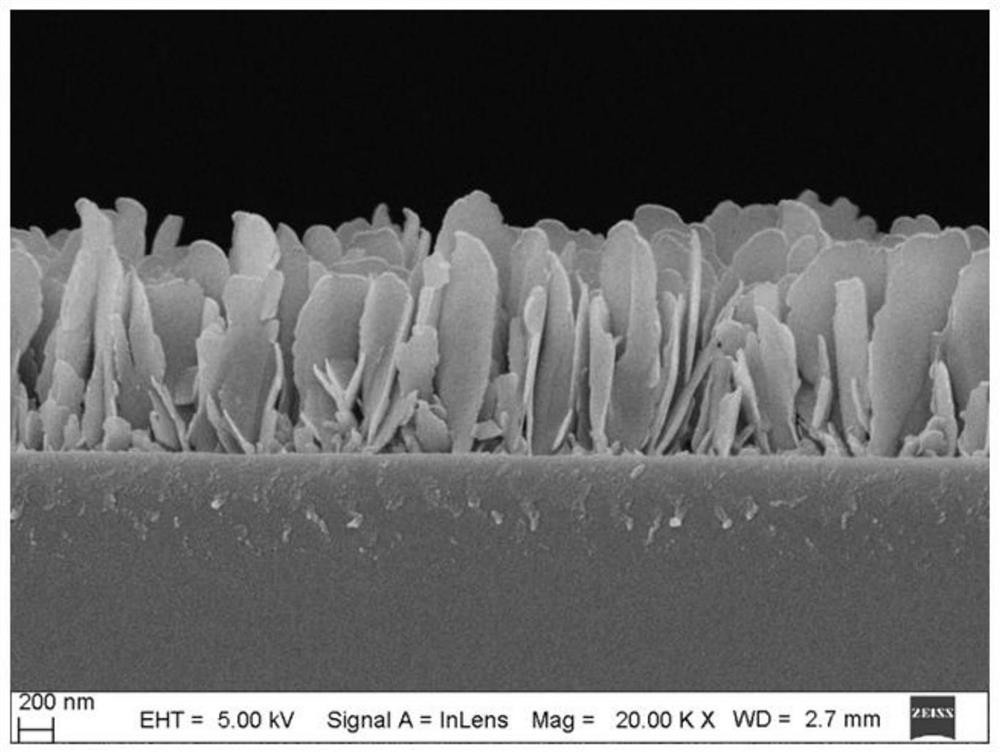
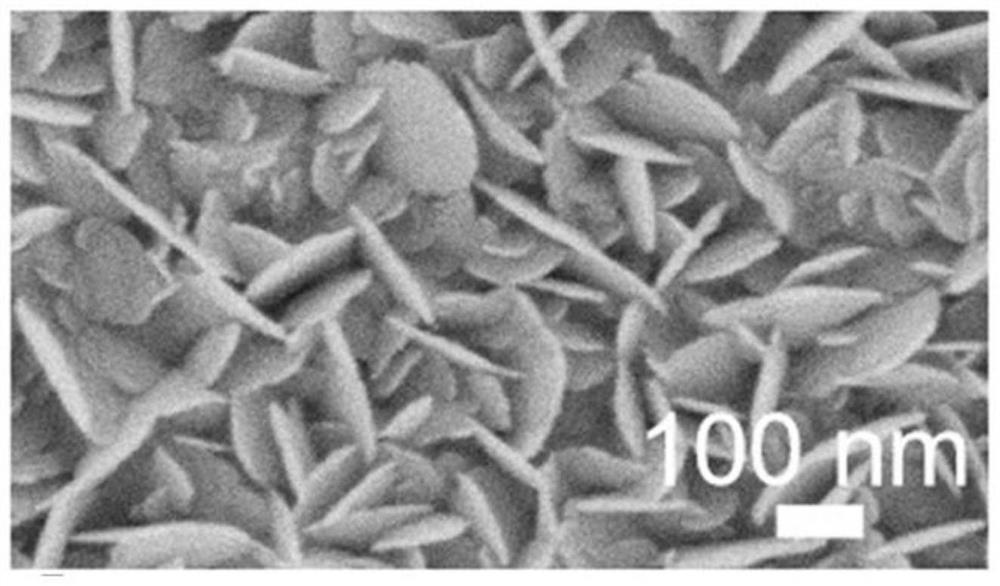
Surface-enhanced Raman scattering substrate, preparation method and application thereof
InactiveCN111896523AStrong chemical enhancementWith physical enhancementRaman scatteringHeterojunctionPhysical chemistry
The invention provides a surface-enhanced Raman scattering substrate, which comprises: a supporting substrate, a semiconductor nanosheet array vertically growing on the supporting substrate, and a noble metal layer deposited on the semiconductor nanosheet array. The invention further provides a preparation method of the surface-enhanced Raman scattering substrate, and application of the surface-enhanced Raman scattering substrate in detection of Raman signals of a to-be-detected object. According to the invention, the detection function layer comprises a semiconductor-metal heterojunction structure composed of a semiconductor nanosheet array and a precious metal layer, and the semiconductor-metal heterojunction structure not only has a physical enhancement effect, but also has a relativelystrong chemical enhancement effect, so that the structure has very high surface enhanced Raman activity, and has very high stability and repeatability.
Owner:SHENZHEN INST OF ADVANCED TECH
A glutathione reductase assay kit and its preparation method and application
ActiveCN108828215BNo reconstitution requiredImprove accuracyMaterial analysisPreservativeGlutathione reductase
The invention provides a glutathione reductase assay kit, which comprises a reagent R1 and a reagent R2. The reagent R1 includes: 50-200 mmol / L of a Tris buffer solution, 0.5-2 mmol / L of EDTA, 1-5 kU / L of pyruvic carboxylase, 1-5 kU / L of ascorbic acid oxidase, 5-20 mg / L of potassium ferrocyanide, a surfactant and a preservative; the reagent R2 includes: 50-200 mmol / L of the Tris buffer solution, 0.5-2 mmol / L of EDTA, 2-10 mmol / L of GSSG, 0.1-0.5 mmol / L of NADPH, 1-15 g / L of a stabilizer, and 0.5-2 g / L of a preservative. The liquid assay kit has good stability and is strong in anti-interferenceeffect. The invention also discloses a preparation method and an application of the assay kit.
Owner:中拓生物有限公司
Kit for enzyme-linked immunosorbent assay of bovine abortion clamydia
ActiveCN108802368ALong storage timeStrong specificityMaterial analysisImmunosorbentsImmuno detection
The invention relates to the field of immunology, and particularly discloses a kit for enzyme-linked immunosorbent assay of bovine abortion clamydia. The kit realizes enzyme-linked immunosorbent assayof the bovine abortion clamydia by artificially synthesizing polypeptide as shown in SEQ ID NO.1, taking the polypeptide as antigen and coating a solid phase carrier to the polypeptide. The kit takesthe artificially synthesized polypeptide antigen as coating antigen, and ensures that enzyme-linked immunosorbent assay has the characteristics of high specificity and good sensitivity. The kit further provides an excellent antigen protective agent after the antigen is coated with the solid phase carrier, protects the coated antigen, and prolongs the storage time of the antigen.
Owner:CHINA AGRI UNIV
Methyl esterification luminol enzyme-catalyzed chemical luminescence substrate system
InactiveCN101526479AWide detection rangeHigh sensitivityChemiluminescene/bioluminescenceLuminous intensityOxygen
The invention discloses a methyl esterification luminol enzyme-catalyzed chemical luminescence substrate system, which consists of a reagent A and a reagent B, wherein the reagent A consists of 0.90 mM of methyl esterification N-(4-amino butyryl)-N-ethyl-iso-luminol and 0.01 to 0.05 M of a carbonate buffer solution with the pH value of 9.6; and the reagent B consists of 0.01 to 0.05 M of a carbonate buffer solution of with the pH value of 9.6, 0.05 to 0.1 mM of urea peroxide and 0.15 to 0.30 mM of peroxy phenol. In 4 to 6 magnitudes, the luminous intensity of the methyl esterification luminol enzyme-catalyzed chemical luminescence substrate system shows a good linear relation with the concentration of measuring substances, and in a wider testing range, the methyl esterification luminol enzyme-catalyzed chemical luminescence substrate system can ensure high sensitivity and completely meet requirements on a clinical test.
Owner:TIANJIN CHUANHE TRADE
Test strip and method for detecting prostate tumor antigens
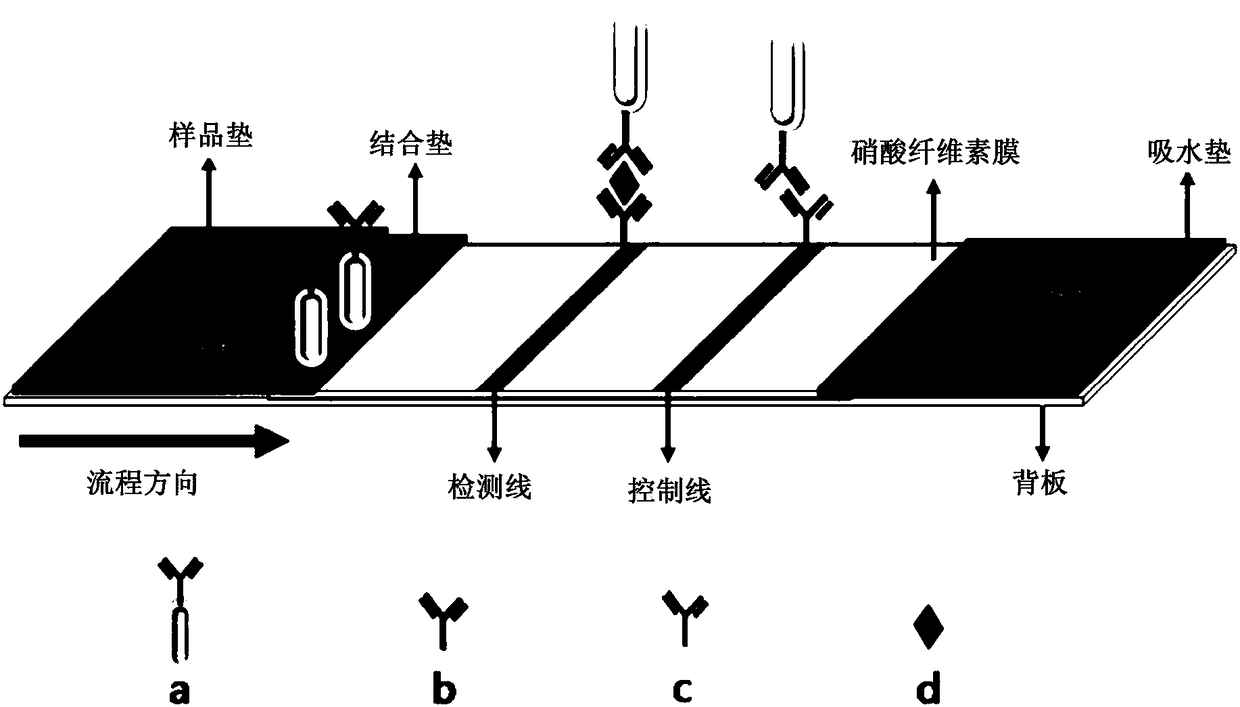
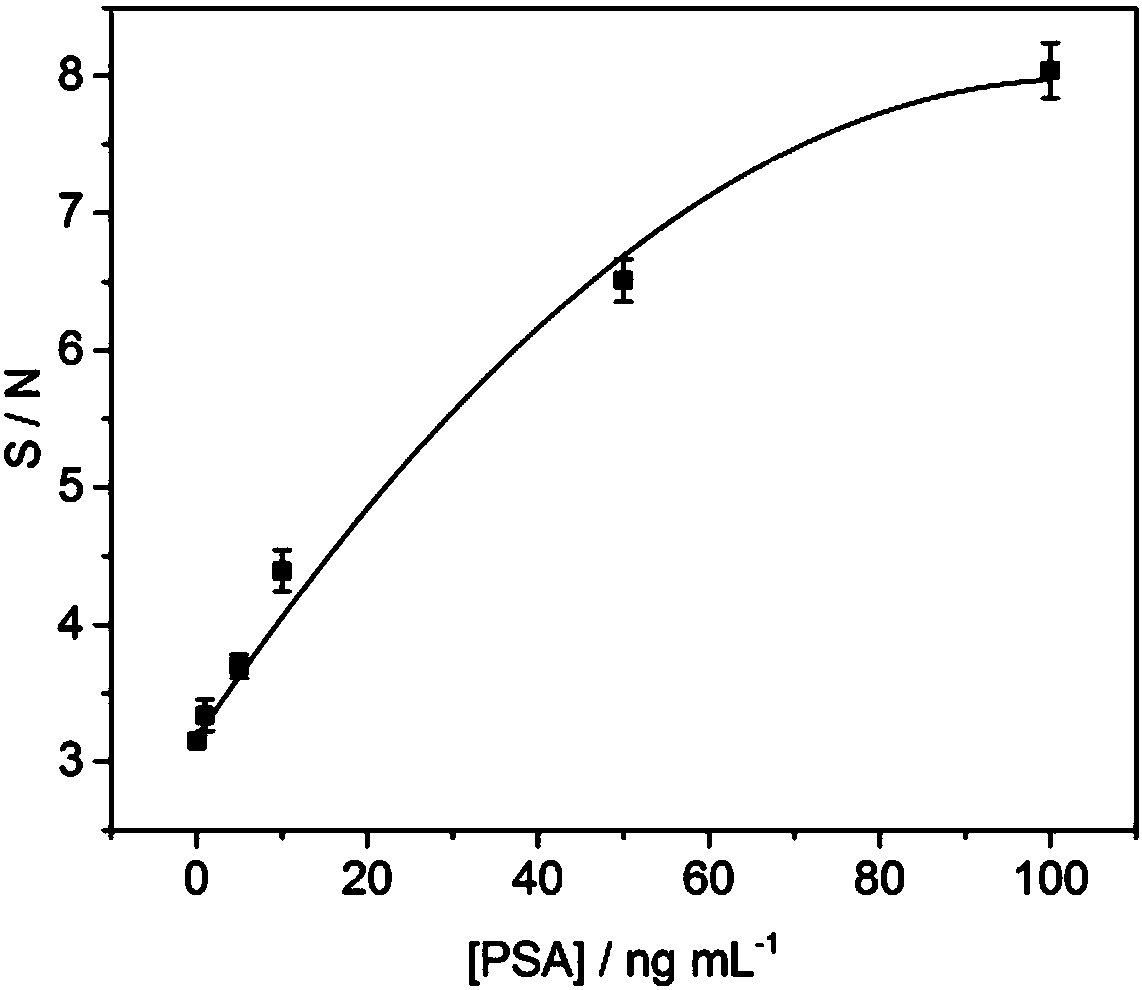
The invention provides a test strip and a method for detecting prostate tumor antigens. By the aid of the test strip and the method, the shortcomings of complicated operation and long time consuming of existing methods for detecting PSA (prostate specific antigens) and difficulty in meeting POC (point of care) quick diagnosis requirements can be overcome. PSA monoclonal antibodies II are labeled by gold nanorods by the aid of immunochromatography technologies, a nitrocellulose membrane is coated with goat anti-rat IgG and PSA monoclonal antibodies I, and the test strip for detecting the PSA isprepared by the aid of double-antibody sandwich processes. Liquid can flows through a binding pad and the nitrocellulose membrane step by step under capillary actions after PSA sample solution is added onto a sample pad of the test strip, and is bound with biological molecules, which are immobilized on the test strip, under antigen-antibody interaction, and a detection line visible to naked eyescan be generated. The test strip and the method are combined with microarray scanners, so that corresponding detection signal strength values can be obtained, and the PSA can be detected. The test strip and the method have the advantages that the method is simple and speedy, and is low in sample consumption and cost and short in detection time, the minimum concentration of the detectable PSA is 0.1 ng mL<-1>, the detection range is 0.1-100 ng mL<-1>, and clinical detection requirements can be met.
Owner:上海格荣生物科技有限公司
Cyanogens-free hemolytic agent for hemocyte analyzer
The invention relates to a cyanogens-free hemolytic agent for hemocyte analyzer. One litre of the cyanogens-free hemolytic agent comprises the following components: 0.8-5.0 g of potassium chloride, 0-60.0 g of dodecyl trimethyl ammonium chloride, 0-14.0 g of octadecyl trimethyl ammonium bromide, 6.0-10.0 ml of isopropanol, a carbonate or hydrochloric acid buffer solution, and the balance of water, wherein the carbonate or hydrochloric acid buffer solution is used for regulating pH to 3.2-5.8; the addition quantities of the dodecyl trimethyl ammonium chloride and the octadecyl trimethyl ammonium bromide are complementary, but the sum of the addition quantities of the dodecyl trimethyl ammonium chloride and the octadecyl trimethyl ammonium bromide is less than or equal to 60 g. The invention can form stable hemoglobin derivatives and meet the requirements of clinical examination because absorption spectrum curves are similar when lambda is equal to 540 nanometers and lambda is equal to 504 nanometers; and in addition, the invention can effectively improve the work environments of operators and reduce the injury of poisons on human body health because reagents do not contain cyanides and trinitrides or have no toxicity.
Owner:南昌百特生物高新技术股份有限公司
Primer and probe combination for detecting methylation of genes related to high-grade cervical lesions and cervical cancer
ActiveCN113265456AImprove forecast accuracyHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesCervical lesionMedicine
The invention discloses a primer and a probe for detecting methylation degrees of genes related to high-grade cervical lesions and cervical cancer. The primer and the probe are used for detecting methylation of sites 425, 427 and 438 of the human EPB41L3 gene, methylation of sites 6367 and 6389 of the viral HPV16L1 gene and methylation of sites 4256, 4261, 4265, 4269, 4275 and 4282 of the HPV18L2 gene. Through a large amount of screening and optimization in the invention, the detection limits of the finally obtained methylation-specific primer and probe on the EPB41L3 gene, the HPV16L1 gene and the HPV18L2 gene are all within 1%. According to the invention, the problem that an existing methylation detection result is insufficient in correlation with high-level cervical lesions and cervical cancer is solved, a more accurate and effective technology for auxiliary diagnosis, scattered management and prognosis judgment of the high-level cervical lesions and cervical cancer is achieved, and cervical cancer screening efficiency is improved.
Owner:苏州凯爱健康科技有限公司
Dry type biochemical analyzer
InactiveCN103994974ASolve difficult-to-collect problemsHigh precisionMaterial analysis by optical meansGratingBeam splitting
The invention discloses a dry type biochemical analyzer belonging to the technical field of biochemical detection equipment. The dry type biochemical analyzer is characterized in that in a mechanical system, a dry sheet sampling position is arranged on an X and Y two-dimensional linear movement platform, two pushing rods are respectively arranged in an X direction of the X and Y two-dimensional linear movement platform, an incubation groove is fixed in front of the pushing rods, a baffle block is arranged in the front end of the incubation groove, and a pushing rod is arranged in a Y direction of the X and Y two-dimensional linear movement platform. In an optical system, (a), an LED (Light Emitting Diode) light source is arranged in an integrating sphere, a lens groove is arranged on an optical axis line of the integrating sphere and a dry sheet; and (b), a rear beam-splitting optical system is located at the underface position vertical to the dry sheet, and a lens group and a grating are arranged on a lower vertical line of the dry sheet. The dry type biochemical analyzer has the beneficial effects that 1, by applying an integrating sphere technology, the problem of difficulty in acquiring a signal due to diffuse reflection is solved, and the precision of an instrument is greatly improved; 2, the instrument is more flexible and is convenient to use; 3, by adopting a rear beam-splitting design, the detection accuracy is improved, and the stability of unit is higher; and 4, the dry type biochemical analyzer is low in cost, small and portable, and high in detection speed and performs detection for 120 tests per hour by applying a multi-channel means; and multiple reagent dry sheets are matched with the instrument, thus the demand of clinical testing is met.
Owner:CHANGCHUN UNIV OF SCI & TECH
An Enzymatic Chemiluminescent Substrate for Alkaline Phosphatase
ActiveCN104990912BHigh strengthLow costChemiluminescene/bioluminescenceBiological testingFluorescenceSolvent
The present invention relates to an alkaline phosphatase enzymatic chemiluminescent substrate. The enzymatic chemiluminescent substrate uses water as a solvent, and also comprises the following components: 2-amino-2-methyl-1-propanol; AMPPD; a luminescence enhancer which is sodium fatty alcohol polyoxyethylene ether carboxylate coupled with a fluorescent compound. The luminescence enhancer has a co-surfactant effect, allowing the complex to be better combined into a chemiluminescent buffer system, thereby significantly increasing the chemiluminescent efficiency. The alkaline phosphatase enzymatic chemiluminescent substrate has advantages such as high strength, high sensitivity, a long duration and good stability, and fully satisfies clinical detection requirements. A main performance indicator of the chemiluminescent substrate reaches a foreign product level, and the costs of the chemiluminescent substrate are significantly reduced.
Owner:SUZHOU HAOOUBO BIOPHARML
Circulating Tumor Cell Capture System
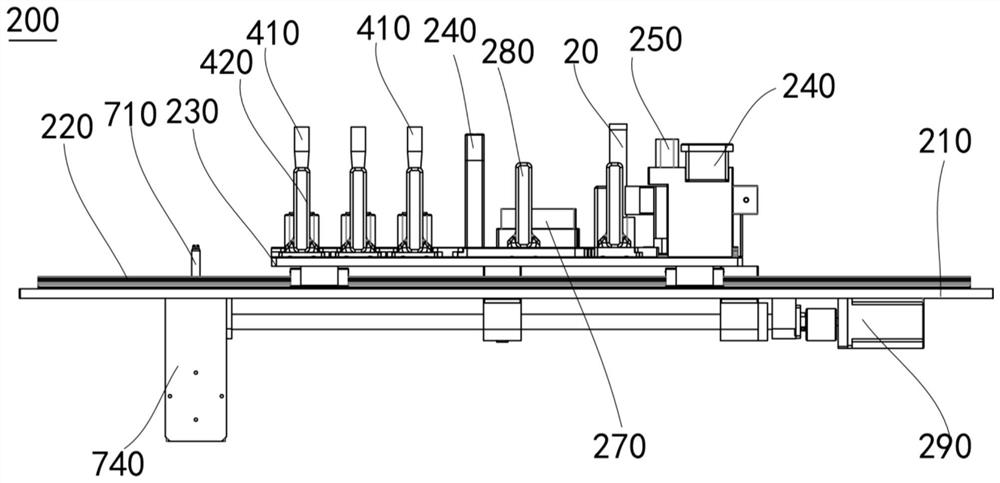
ActiveCN108220152BImplement parallel detectionAvoid influencing factorsCell dissociation methodsBioreactor/fermenter combinationsCirculating cancer cellSurgery
The invention discloses a circulating tumor cell capture system. The circulating tumor cell capture system includes a supporting device, a plugging device, a filtering device, a scanning device, a pipetting device and a suction filtering device. The support device has a support seat and a base plate, and the base plate is slidably connected to the support seat; the plug removal device is used to remove the plug on the sample tube; the filter device has a filter; the scanning device It is used to scan and record the information marking code of the sample tube and the information marking code of the filter; the pipetting device is used to suck the sample liquid from the sample tube and send it to the filter; the suction filtering device has a suction head and Suction filter, the suction filter suction head is arranged on the support base for the connection of the filter, the suction filter is communicated with the suction filter suction head for the filter suction head Suction filter described above. The circulating tumor cell capture system has the advantages of simple operation, good stability and high sensitivity.
Owner:SUREXAM BIO TECH
Protein C activity determination kit based on chromophoric substrate method
InactiveCN114814245AHigh sensitivityStrong linear rangeMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementZymogenChromogenic Substrates
The invention discloses a protein C activity determination kit based on a chromophoric substrate method, protein C is a vitamin K dependent plasma serine protease zymogen, and comprises a reagent R1, a reagent R2 and a diluting reagent; the R1 reagent comprises a protein C activator, a first buffer solution and a first auxiliary material; the R2 reagent comprises a chromogenic substrate Pca-5297, a second buffer solution and a second auxiliary material; the diluting reagent comprises a third buffer solution and a third auxiliary material, and the pH of the first buffer solution, the pH of the second buffer solution and the pH of the third buffer solution are 7.2-7.6; the protein C activator and the chromophoric substrate Pca-5297 are matched for use, during detection, the cutting efficiency is high, the reaction signal is strong, the reaction speed is high, the sensitivity is high, the linear range is wide, the reaction time is short, the sample distinction degree is increased, and establishment of the linear range and clinical sample testing are facilitated; the reagent is liquid, convenient to use, low in cost and good in stability.
Owner:SHENZHEN DYMIND BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com