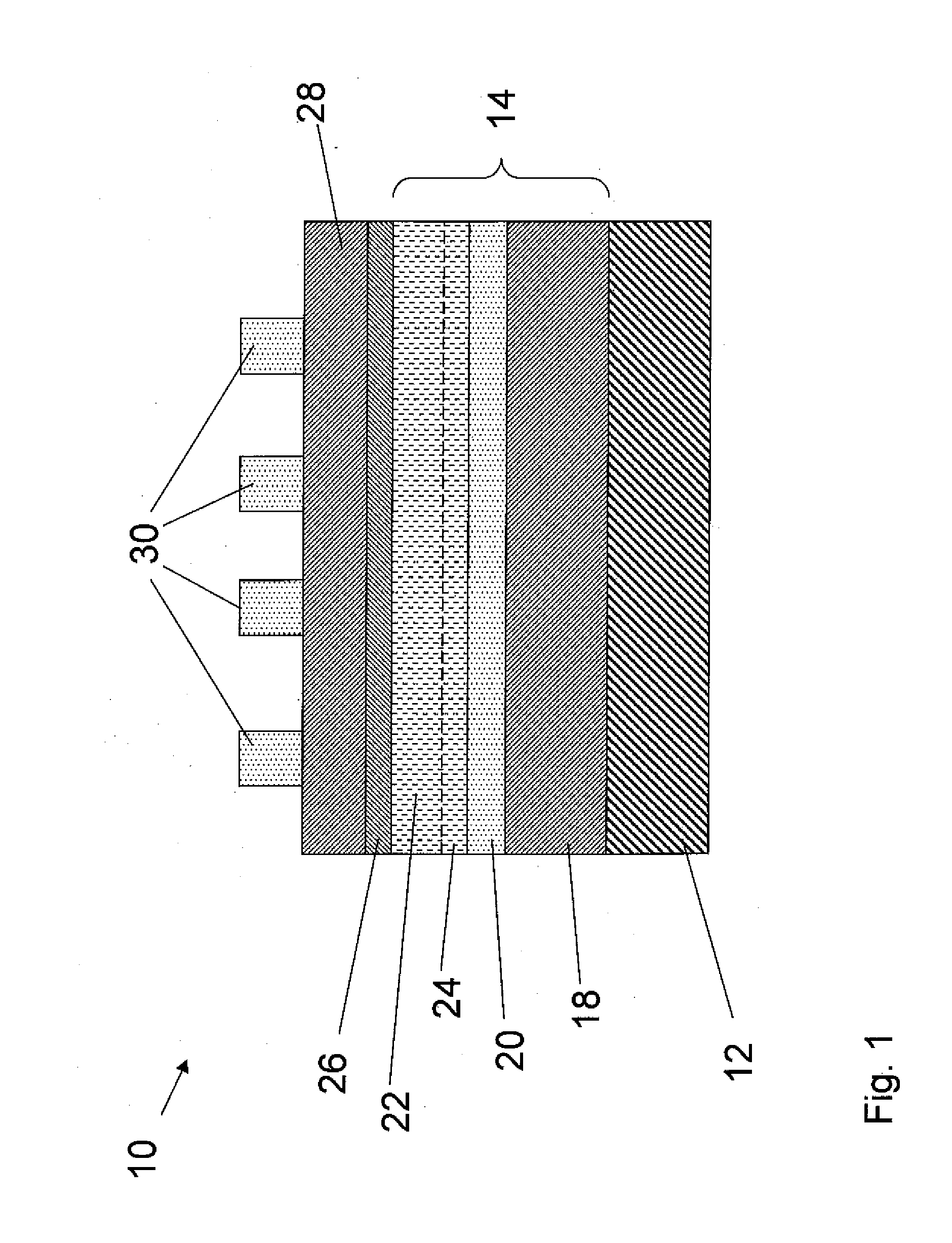
Patents
Literature
87 results about "Zinc phosphide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
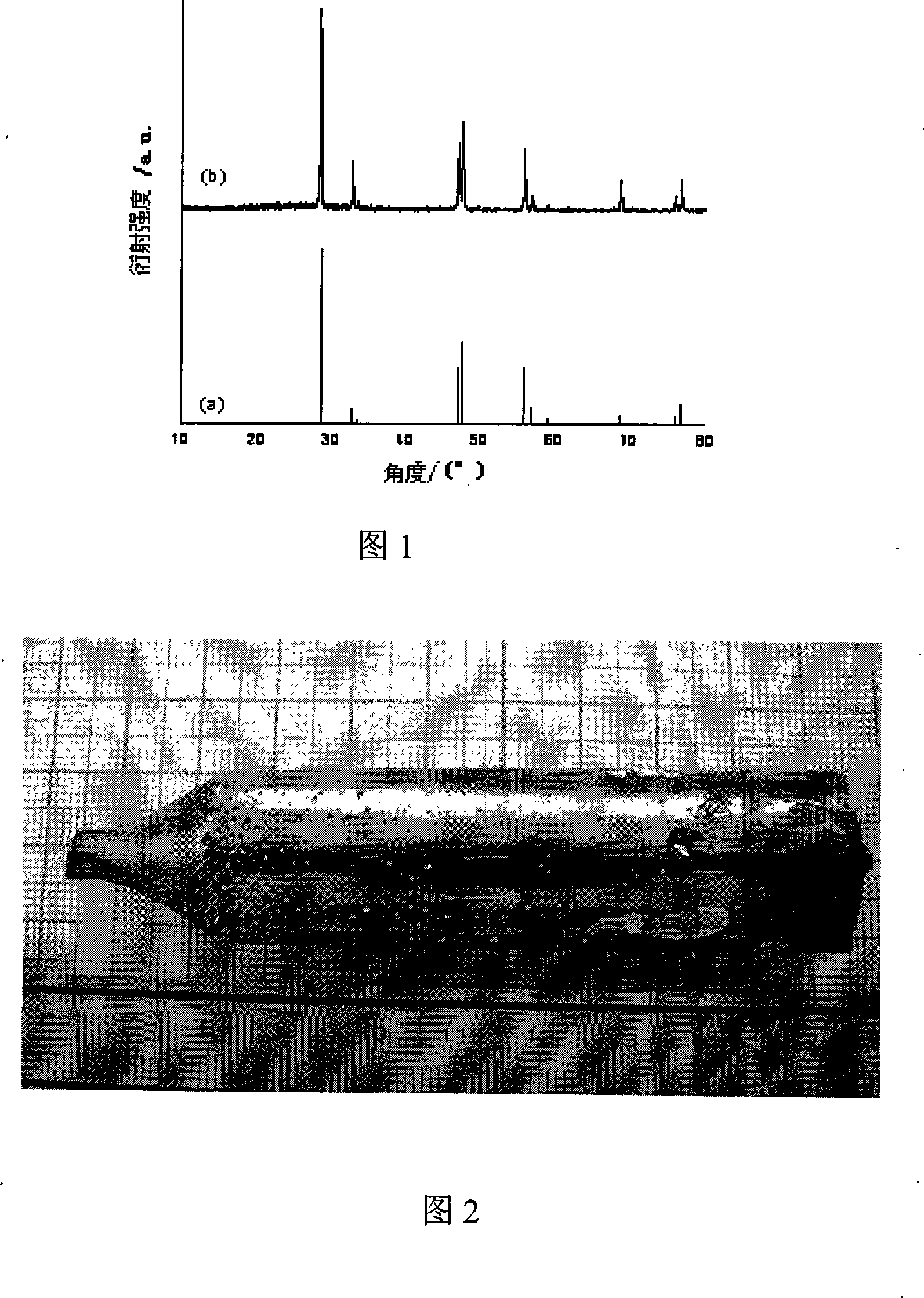
Zinc phosphide (Zn₃P₂) is an inorganic chemical compound. It is a grey solid, although commercial samples are often dark or even black. It is used as a rodenticide. Zn₃P₂ is a semiconductor with a direct band gap of 1.5 eV and may have applications in photovoltaic cells. A second zinc phosphide is known, with the stoichiometry ZnP₂.
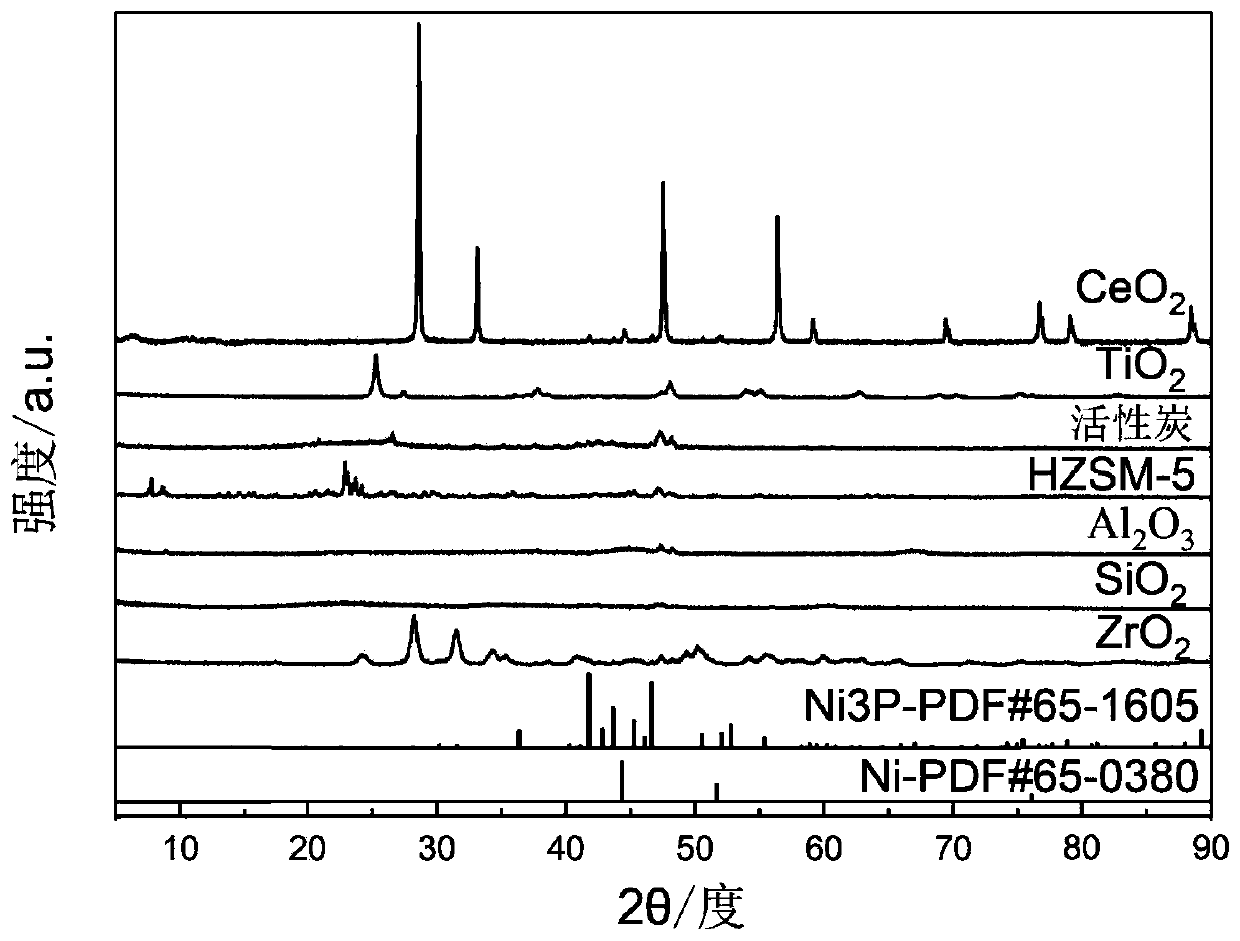
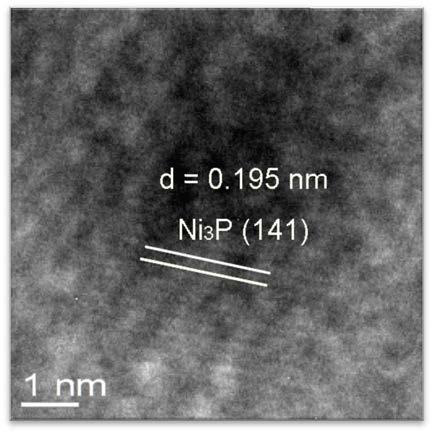
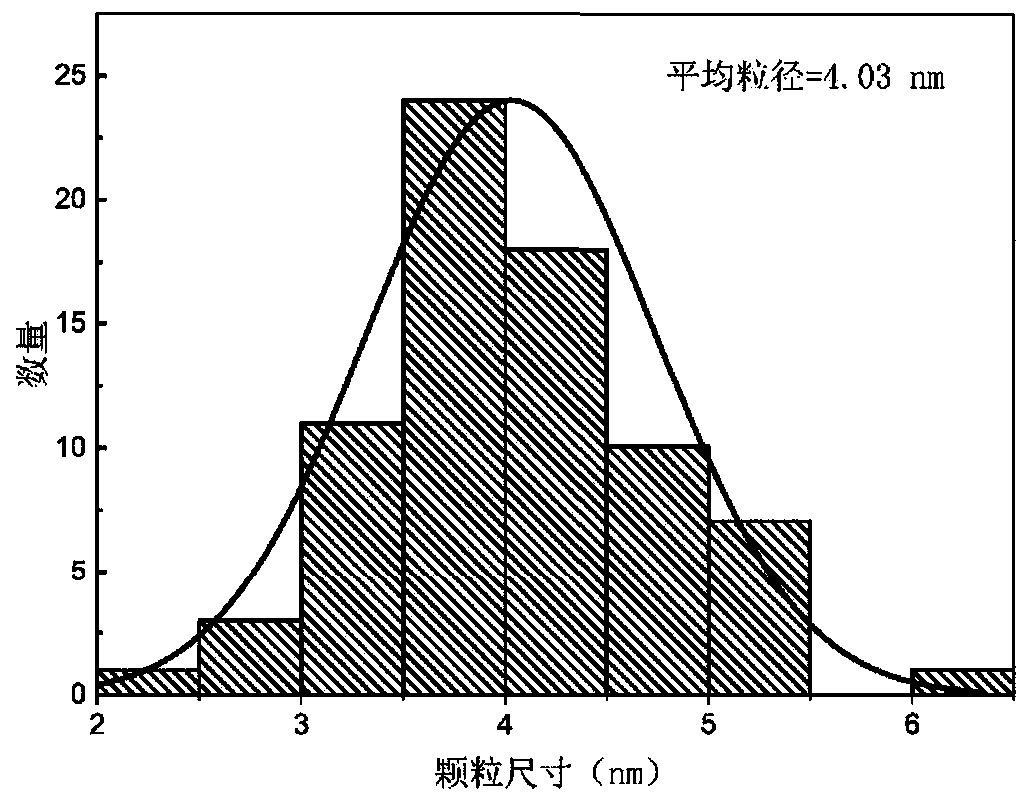
Preparation method of nickel phosphide
InactiveCN101734633ASolve the problem of sulfur poisoningIncrease investmentPhosphidesDispersityPhosphorous acid
On the premise of deep hydrodesulfurization, the main problem in diesel fuel's hydrotreatment is to rid the refractory sulfide and hydrogenate polycyclic aromatic hydrocarbon. Loaded nickel phosphide catalyst demonstrates higher hydrodesulfurization activity and better activity stability. However, the traditional nickel phosphide catalyst is prepared by temperature programming of nickel phosphide, so that dispersion degree of active components is low. In the invention, through solid-phase reaction between phosphorous acid ions and nickel ions, bulk phase and loaded nickel phosphide catalyst with higher active component dispersion degree and fine catalytic activity are obtained at low temperature. The invention is applicable to diesel oil hydroprocessing.
Owner:NANJING UNIV
High density spherical nano lithium iron phosphate material, a preparation method thereof, and lithium ion battery containing same
ActiveCN106229505ATightly boundImprove liquidityCell electrodesSecondary cellsSodium-ion batteryLithium-ion battery
A high density spherical nano lithium iron phosphate material, a preparation method thereof, and a lithium ion battery containing the same are provided. The preparation method comprises the following steps: (1) pre-sintering a lithium resource, iron phosphides, adulterant, and a carbon source with protective gas after dry mixing; (2) mixing a pre-sintering material, dispersant, and deionized water, and performing ultra fine grinding; (3) performing spray drying on a sizing agent obtained by ultra fine grinding, to obtain a spherical nano lithium iron phosphate precursor; and (4) performing chemical vapor deposition coating on the spherical nano lithium iron phosphate precursor obtained in step (3), to prepare the high density spherical nano lithium iron phosphate material. A primary particle size of the lithium iron phosphate material prepared in the present invention is not large, and a powder conductivity may reach 10.1 S / cm, so that a material capacity, low temperature, rate performance, and fabrication and cycling performance may be well balanced.
Owner:江苏贝特瑞纳米科技有限公司
Polycrystalline synthesis and single-crystal growth method for germanium zinc phosphide
InactiveCN101235542AIncrease synthesis rateHigh purityPolycrystalline material growthFrom solid stateForeign matterCrucible
A method for synthesizing poly-crystal and growing single-crystal of zinc germanium phosphide relates to a method for synthesizing and the growing the zinc germanium phosphide. The method solves the problem of low synthesizing rate of the current method for synthesizing the zinc germanium phosphide poly-crystal, and the problem of uneasy to exclude foreign matter of the method for growing the zinc germanium phosphide single-crystal. The synthesis of the zinc germanium phosphide poly-crystal of the invention comprises the following steps: firstly, defining the quantity, secondly, increasing the temperature, namely obtaining the zinc germanium phosphide poly-crystal material; the growth of the zinc germanium phosphide single-crystal protocaryon of the invention has the following steps: firstly, defining the quantity, secondly, increasing the temperature, thirdly, decreasing the copple and reducing the temperature, namely obtaining the zinc germanium phosphide single-crystal, the growth of the zinc germanium phosphide single-crystal with seed crystal of the invention has the following steps: firstly, defining the quantity, secondly, increasing the temperature, thirdly, decreasing the copple and reducing the temperature, namely obtaining the zinc germanium phosphide single-crystal. The method of the invention has the advantages that the synthesizing rate of the poly-crystal synthesis is high, the growth of the single-crystal is easy to exclude the foreign matter, and the direction of the crystal is accordant.
Owner:HARBIN INST OF TECH
Novel method for preparing nickel phosphide by low-temperature reduction
The invention provides a novel method for preparing nickel phosphide (Ni12P5) by the low-temperature solvothermal method. The method of the invention is characterized by dissolving anhydrous nickel chloride as a nickel source and sodium hypophosphite as a phosphorus source in a high-boiling-point organic solvent at the room temperature, stirring the materials until the organic solvent is transparent, sealing the organic solvent in a high-pressure kettle and heating up to 140-240 DEG C and reacting for certain time to obtain Ni12P5. The method of the invention is characterized by using a high-boiling point organic substance as the solvent, the reaction conditions are simple and mild and the generated Ni12P5 is the nano-particle with the size being 50nm to 200nm and the specific surface area being larger. The method is favorable for hydrodesulfurization, selective hydrogenation and other catalytic hydrogenation reactions, thus having wider application range.
Owner:NANKAI UNIV
Preparation method of nickel phosphide catalyst carried by composite carrier
InactiveCN101612584ALittle loss of activityHigh activityCatalyst activation/preparationRefining to eliminate hetero atomsNickel saltPhosphate
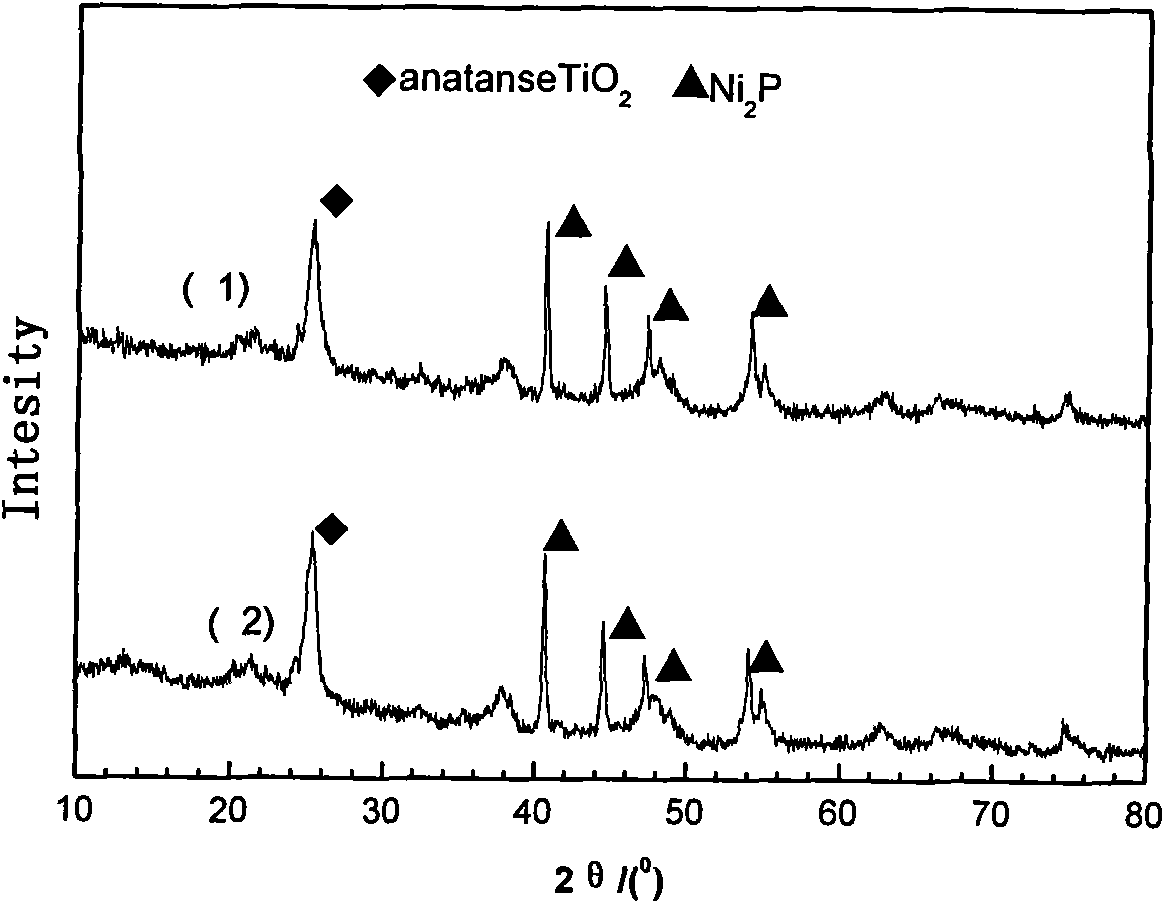
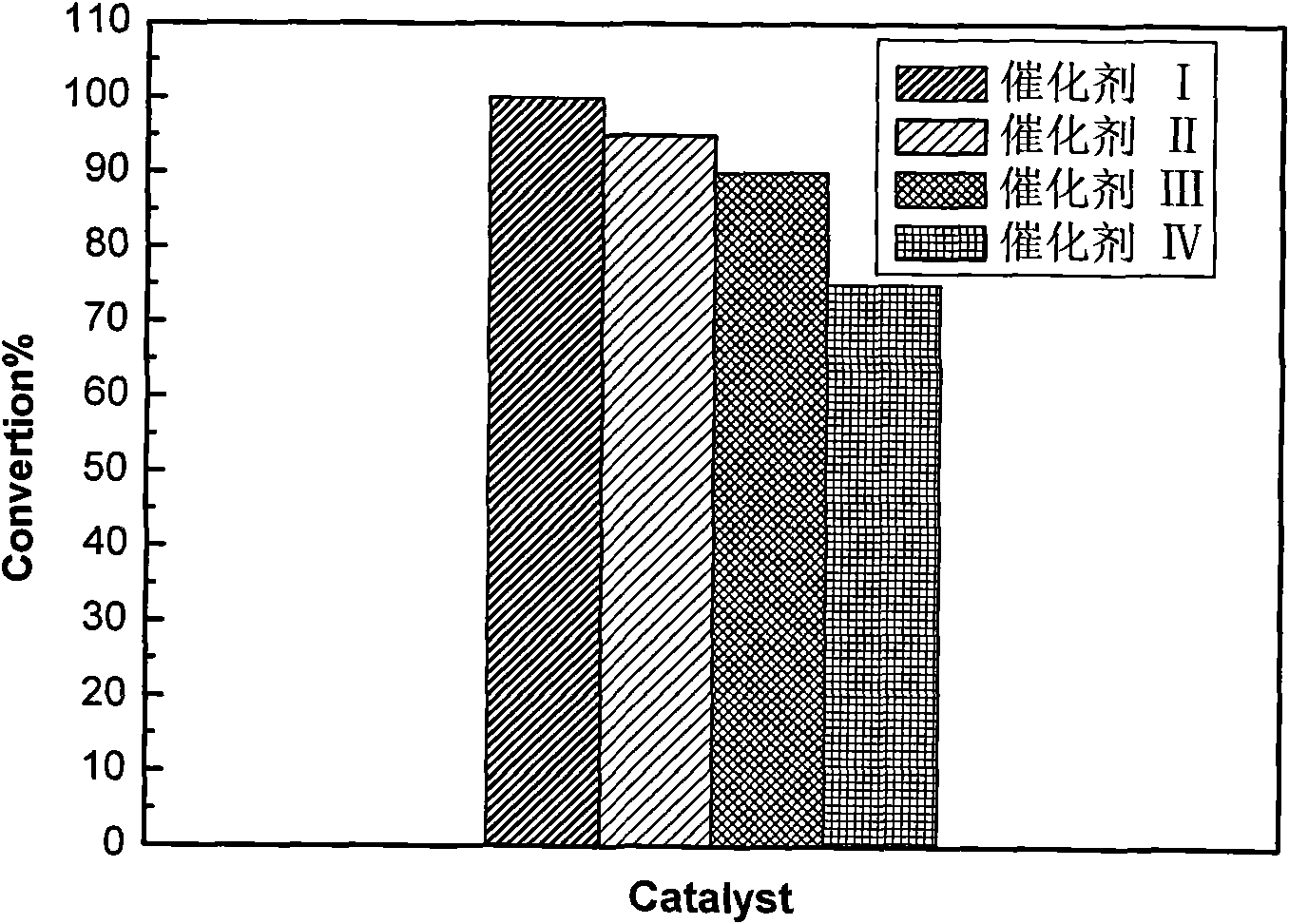
The invention relates to a preparation method of a nickel phosphide catalyst carried by a composite carrier. The method is characterized by comprising the following steps: firstly, using activated alumina and relative enzymes and alcohols as raw materials to prepare a composite carrier of TiO2-Al2O3 by adopting an improved sol-gel method; further, selecting suitable nickel salt and phosphate and carrying out ultrasound dipping and drying in microwaves to obtain a catalyst precursor; and finally, increasing a temperature by a program and reducing to obtain the composite support. The composite carrier of TiO2-Al2O3 prepared by the invention not only can effectively overcome the defect that nickel phosphide is apt to react with gamma-Al2O3 to generate AlP4 to lose the activity, but also overcomes the defect that a specific surface area of single TiO2 is relatively small, effectively exerts the strong metal-support interaction (SMSI) of the TiO2, thereby obtaining a hydrodesulfurization catalyst with remarkable activity efficacy.
Owner:XI AN JIAOTONG UNIV
Method for preparing zinc phosphide/cobalt phosphide heterojunction photocatalyst based on MOFs template
InactiveCN106311294AImprove photocatalytic hydrogen production performanceFully exposedPhysical/chemical process catalystsHeterojunctionPtru catalyst
The invention provides a method for preparing a zinc phosphide / cobalt phosphide heterojunction photocatalyst based on an MOFs template and belongs to the technical field of preparation of photocatalytic materials. ZIF-ZnCo is used as a template, and the zinc phosphide / cobalt phosphide heterojunction photocatalyst with a porous structure is formed by conducting oxidation and phosphating treatment on the template. The original framework structure characteristics of a precursor MOFs are kept based on the catalyst, active catalysis sites are fully exposed, meanwhile the catalyst promotes separation of photoproduced electron holes and improves an photo-response behavior through a good synergistic effect, and therefore shows excellent water photolysis hydrogen-production performance.
Owner:BEIJING UNIV OF TECH
Preparation method for nickel phosphide with hollow core-shell structure
The invention relates to a preparation method for nickel phosphide with a hollow core-shell structure. The preparation method comprises the following steps: firstly, adding deionized water in the polytetrafluoroethylene liner of a reaction kettle, weighing soluble nickel salt, adding the soluble nickel salt into the deionized water and stirring by a magnetic stirrer until the soluble nickel salt is dissolved; then adding an anionic surfactant and a cationic surfactant and stirring until the anionic surfactant and the cationic surfactant are completely dissolved; adding elementary substance phosphorus into the solution, then sealing the polytetrafluoroethylene liner into a stainless steel die, heating from the room temperature under the closed condition, moving obtained black precipitates to a centrifuge tube after the reaction is completed, sequentially and repeatedly washing by benzene, water and absolute ethyl alcohol and centrifuging; finally, placing the washed product into a vacuum drying box to obtain the nickel phosphide with the hollow core-shell structure. The prepared nickel phosphide catalyst with the hollow core-shell structure has excellent photocatalytic performance on organic dye, the preparation method is simple to operate, the reaction is easy to control, good repeatability is achieved and heat treatment at the later period is not required.
Owner:SHAANXI UNIV OF SCI & TECH
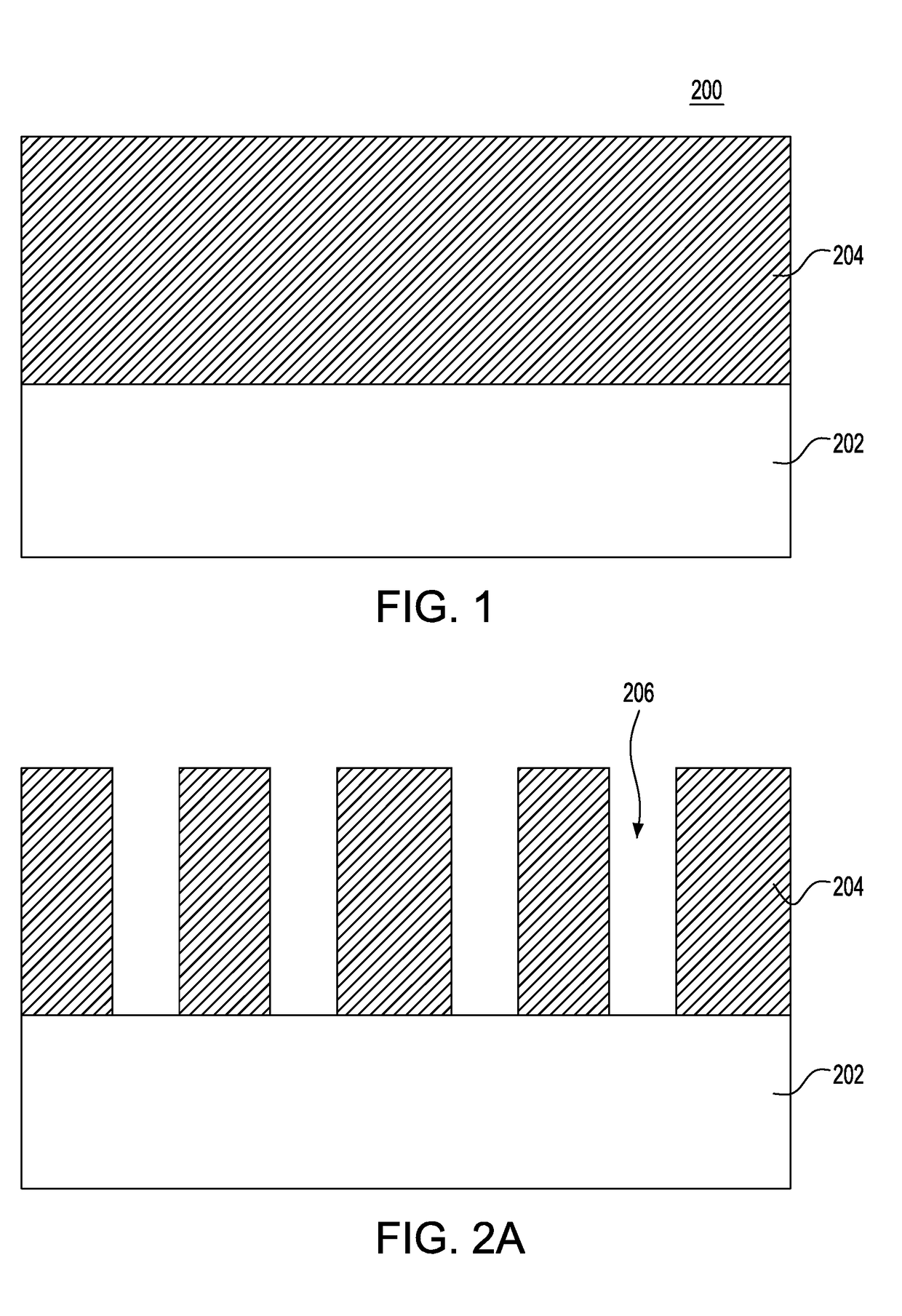
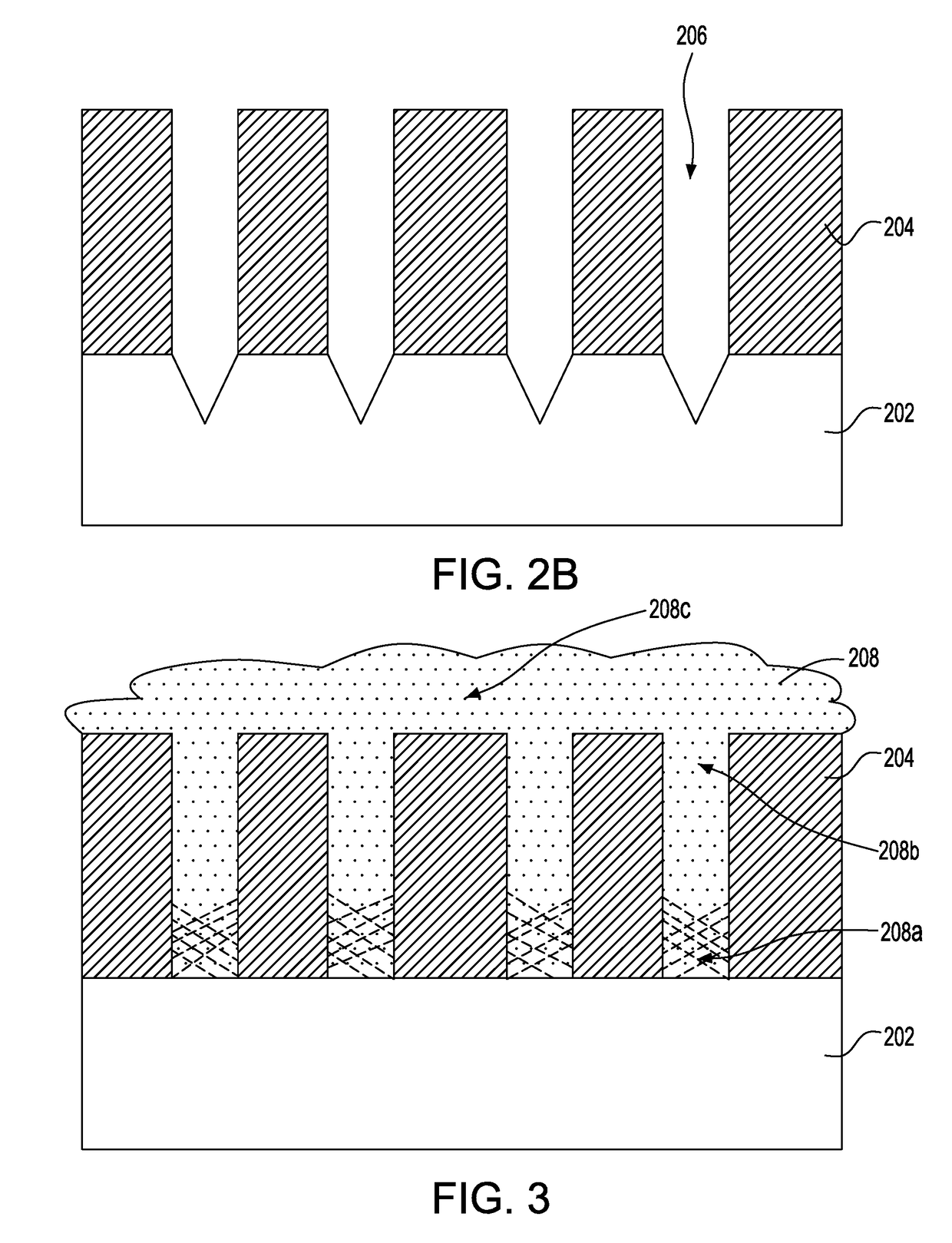
Indium phosphide smoothing and chemical mechanical planarization processes
InactiveUS20170110332A1Suppress generationSemiconductor/solid-state device manufacturingPolishing compositions with abrasivesSlurryPhosphine
A chemical mechanical planarization for indium phosphide material is provided in which at least one opening is formed within a dielectric layer located on a substrate. An indium phosphide material is epitaxially grown within the at least one opening of the dielectric layer which extends above a topmost surface of the dielectric layer. The indium phosphide material is planarized using at least one slurry composition to form coplanar surfaces of the indium phosphide material and the dielectric layer, where a slurry composition of the at least one slurry composition polishes the indium phosphide material selective to the topmost surface of the dielectric layer, and includes an abrasive, at least one pH modulator and an oxidizer, the at least one pH modulator including an acidic pH modulator, but lacks a basic pH modulator, and where the oxidizer suppresses generation of phosphine gas.
Owner:IBM CORP +1
Graded zinc diffusing method based on MOCVD (Metal-Organic Chemical Vapor Deposition) system for producing chip of indium-gallium-arsenic photoelectric detector
ActiveCN101567407AIncrease the carrier concentrationImprove responsivenessFinal product manufactureChemical vapor deposition coatingPressure riseDiffusion methods
The invention relates to a graded zinc diffusing method based on an MOCVD system for producing the chip of an indium-gallium-arsenic photoelectric detector. The method comprises the following steps: a semiconductor wafer which does not form an InGaAs PIN structure of a PN junction is cleaned and blown to dry by high-purity nitrogen; an MOCVD reaction chamber is heated up, depressurized and cleaned; the semiconductor wafer is placed in the reaction chamber; the pressure of the reaction chamber is set; diffusion taking a zinc phosphide compound which is formed by zinc methide (DMZn) and phosphorane (PH3) at high temperature as a diffusion source is conducted with the presence of catalytic reaction and the shielding gas; after the completion of the diffusion, the gas is turned off, the reaction chamber is cooled down and filled with nitrogen and has a pressure rise; and the wafer which undergoes the diffusion is taken out of the reaction chamber. As the diffusion rules of zinc in InP material and InGaAs material have great difference, the graded diffusion method is adopted. With the method, both the InP layer and the InGaAs layer can have rather high current carrier concentration, good interface, rather good homogeneity, technique repeatability, high response and precise control and can be manufactured on a large scale.
Owner:WUHAN HUAGONG GENUINE OPTICS TECH
Method for preparing tetrahedral light-emitting indium phosphide/zinc sulfide core-casing quantum dots
ActiveCN107312534ALow priceStable chemical structureLuminescent compositionsChemical structureQuantum yield
The invention discloses a method for preparing tetrahedral light-emitting indium phosphide / zinc sulfide core-casing quantum dots. The method comprises the following steps: preparing high-boiling point tris(dialiphatic amido) phosphine, preparing InP quantum dot cores, preparing InP / ZnS core-casing quantum dots and separating the InP / ZnS core-casing quantum dots. Through the use of the high-boiling point tris(dialiphatic amido) phosphine as a phosphorus source, the price is low, the chemical structure is stable, the reaction is mild and safe, and gas is not produced. All reaction precursors are common chemical reagents and low in toxin and safe to store and use. The luminescent color of the InP / ZnS core-casing quantum dots can be precisely controlled simultaneously by the reaction temperature, the ratio of indium halide to the tris(dialiphatic amido) phosphine, the type of the halide and the reaction time, and high fluorescence quantum yield can be ensured.
Owner:南京紫同纳米科技有限公司
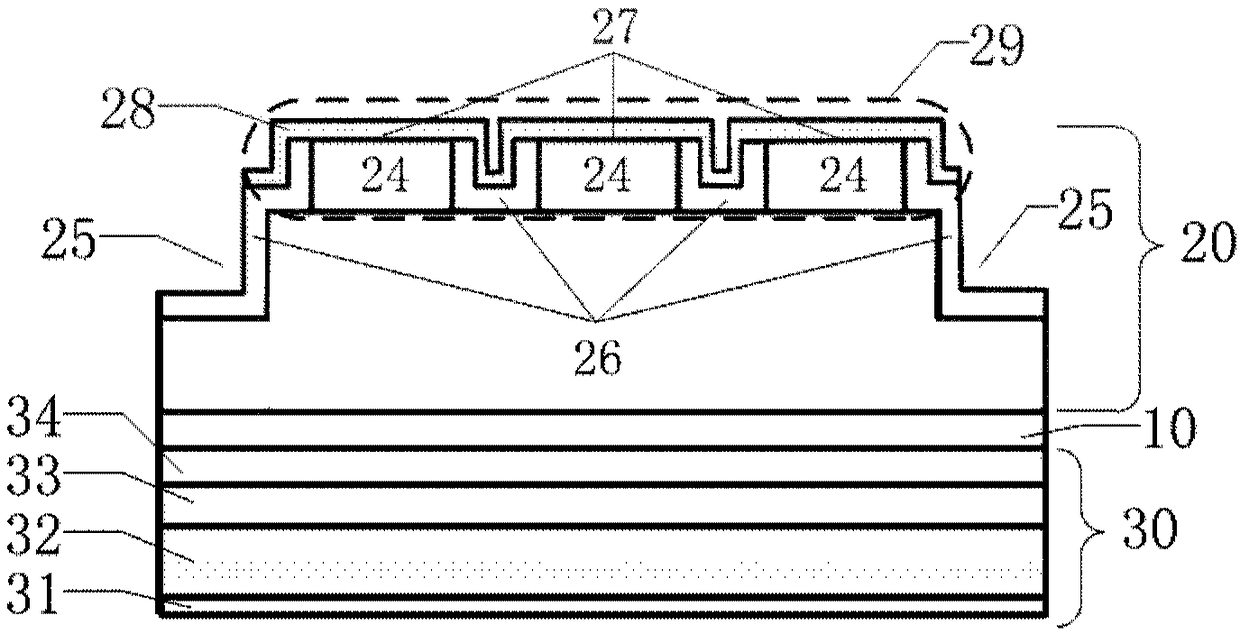
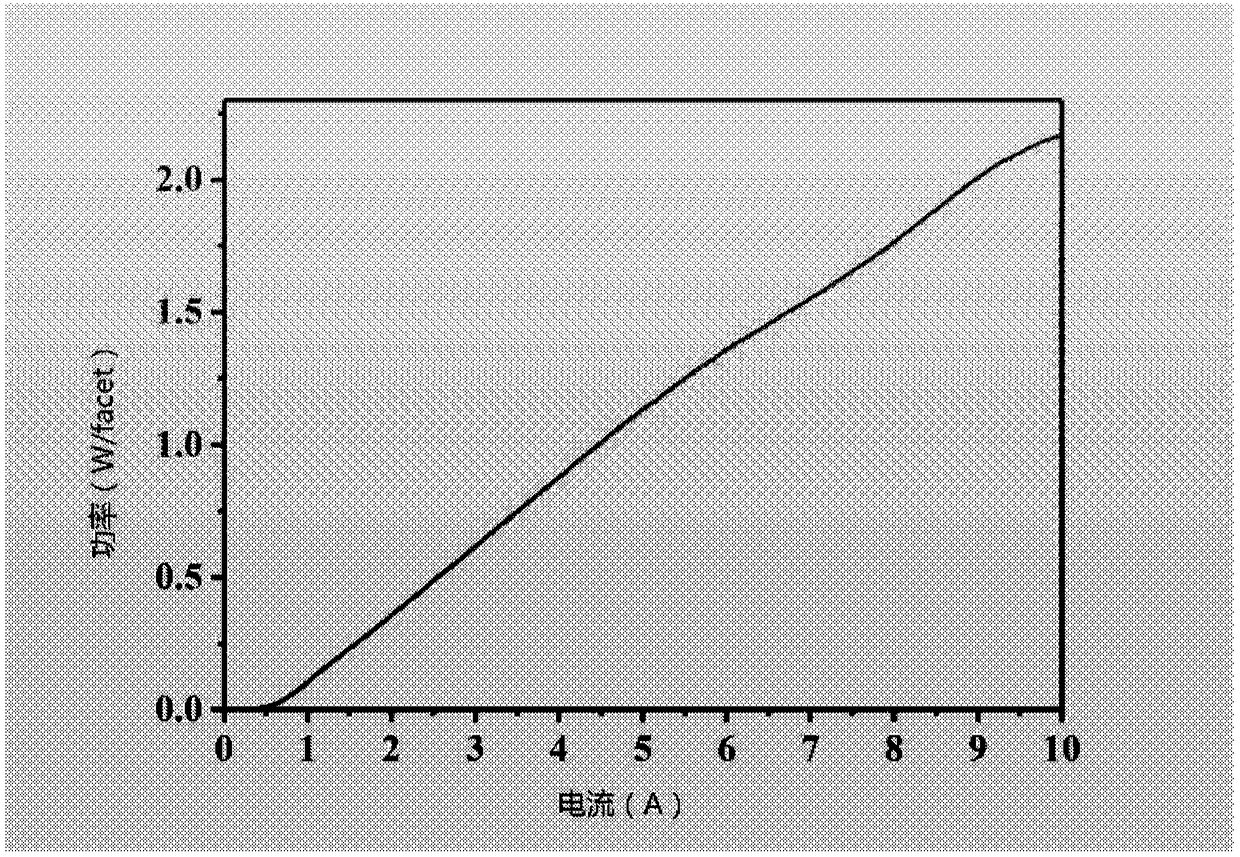
Semiconductor laser based on an indium phosphide-base coupled ridge array and preparation method thereof
InactiveCN108365516AAvoid lasingIncrease the restrictive effectOptical wave guidanceRidge waveguidesQuantum well
The disclosure provides a semiconductor laser based on an indium phosphide-base coupled ridge array. The semiconductor laser is formed by processing an indium phosphide-base epitaxial wafer substrateand comprises a quantum well active layer located in the middle of the semiconductor laser for emitting laser light; a N-region structural layer located below the quantum well active layer for providing electrons and restrictions on carriers and light fields; and a P-region structural layer located above the quantum well active layer for providing holes and restrictions on the carriers and the light fields, wherein the P-region structural layer comprises an coupled ridge array consisting of M ridge waveguides, wherein M is greater or equal to 2, the coupled ridge array enables active region output light fields underneath the adjacent ridge waveguides to achieve coherent coupling and enhances the limitation of the ridge waveguide on the light field in the active region, and the laser is prevented from lasing in a multi-transverse mode, so that the output beam quality is higher.
Owner:INST OF SEMICONDUCTORS - CHINESE ACAD OF SCI
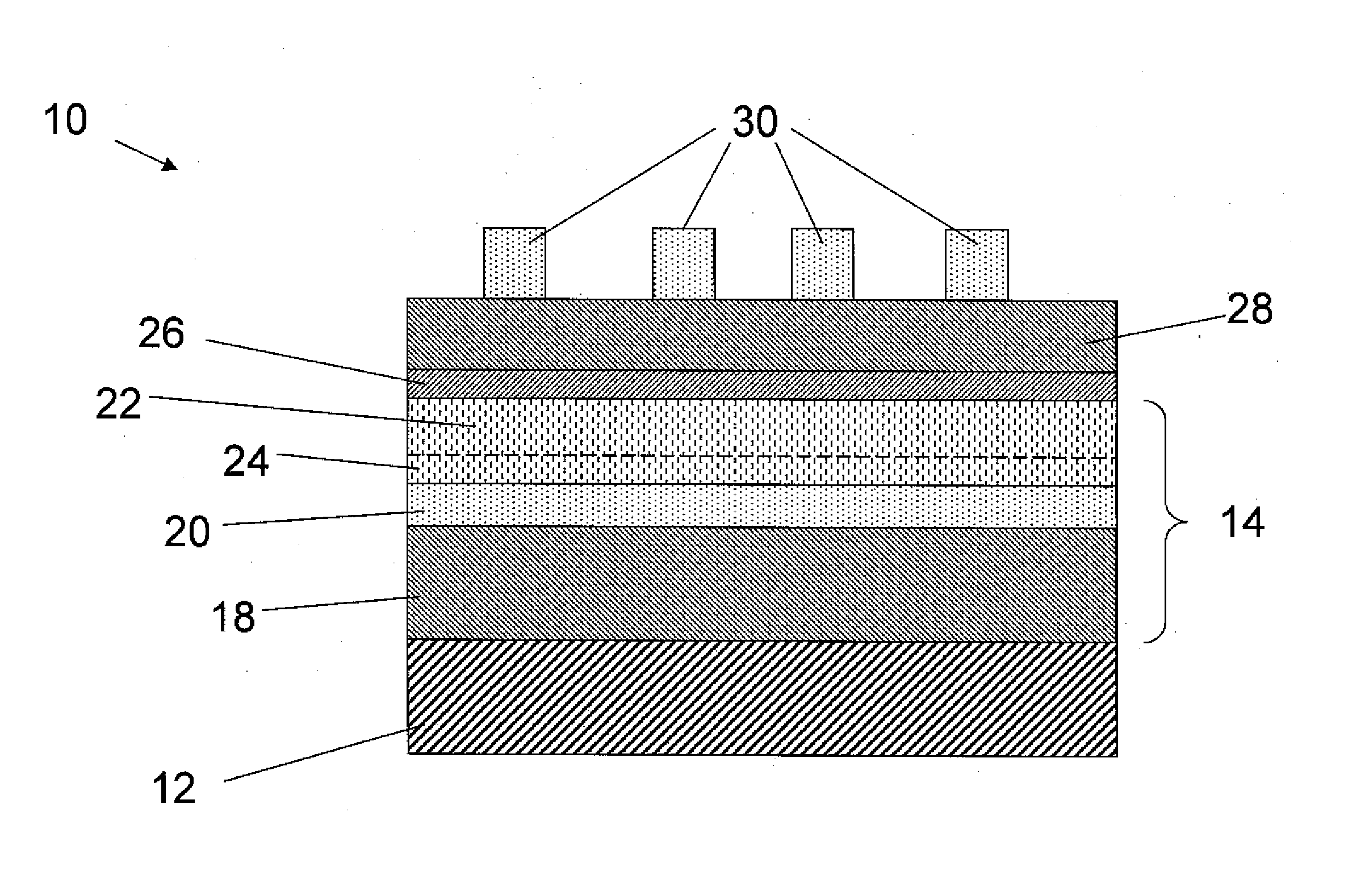
Method of making photovoltaic devices with reduced conduction band offset between pnictide absorber films and emitter films
InactiveUS20160071994A1Reduces conduction bandQuality improvementSemiconductor/solid-state device manufacturingPhotovoltaic energy generationEngineeringAlloy
The principles of the present invention are used to reduce the conduction band offset between chalcogenide emitter and pnictide absorber films. Alternatively stated, the present invention provides strategies to more closely match the electron affinity characteristics between the absorber and emitter components. The resultant photovoltaic devices have the potential to have higher efficiency and higher open circuit voltage. The resistance of the resultant junctions would be lower with reduced current leakage. In illustrative modes of practice, the present invention incorporates one or more tuning agents into the emitter layer in order to adjust the electron affinity characteristics, thereby reducing the conduction band offset between the emitter and the absorber. In the case of an n-type emitter such as ZnS or a tertiary compound such as zinc sulfide selenide (optionally doped with Al) or the like, an exemplary tuning agent is Mg when the absorber is a p-type pnictide material such as zinc phosphide or an alloy of zinc phosphide incorporating at least one additional metal in addition to Zn and optionally at least one non-metal in addition to phosphorus. Consequently, photovolotaic devices incorporating such films would demonstrate improved electronic performance.
Owner:CALIFORNIA INST OF TECH +1
Preparation method of sulfur-doped nickel phosphide nano powder and application in electrolysis of water
The invention provides sulfur-doped nickel phosphide nano powder and an application in electrolysis of water. A method of preparing the sulfur-doped nickel phosphide nano powder comprises the following steps: (1) performing pretreatment on a nickel-containing aqueous solution, adjusting pH of the solution, adding an alkali regulating agent to prepare an alkaline pre-reaction solution, performing areaction under heating, after the reaction is completed, performing washing, performing centrifugation, and performing collection to obtain nickel source precursor powder; (2) performing calcinationtreatment on the nickel source precursor and a phosphating reagent according to a ratio under the protection of an inert gas to obtain nickel phosphide; and (3) performing calcination treatment on thenickel phosphide and a sulfur source under the protection of an inert gas, and performing collection to obtain the black sulphur-doped nickel phosphide powder. The sulfur-doped nickel phosphide provided by the invention has excellent catalytic performance when used for electrocatalysis of an oxygen evolution reaction (OER), has overpotential as low as 0.294 V (relative to a standard hydrogen electrode) and a Tafel slope as low as 58 mV / dec.
Owner:UNIV OF JINAN
Nickel phosphide, preparation method thereof and method for producing hydrogen through water electrolysis
ActiveCN108607586ASimple equipmentEasy to operateCatalyst activation/preparationElectrodesAcetic acidHydrogen
The invention provides nickel phosphide, a preparation method thereof and a method for producing hydrogen through water electrolysis. The preparation method of the nickel phosphide comprises the following steps: firstly, dissolving a nickel source compound, a phosphorus source compound and acetate into water, so as to obtain a dissolving liquid; secondly, performing electrolytic deposition treatment on the dissolving liquid, so as to obtain the nickel phosphide. The preparation method is simple and practicable, mild in condition, does not need severe conditions of high temperature and high pressure, simple in equipment and operation, low in cost, safe and environmentally friendly, does not produce side products, and is suitable for mass production.
Owner:CHONGQING CHANGAN AUTOMOBILE CO LTD
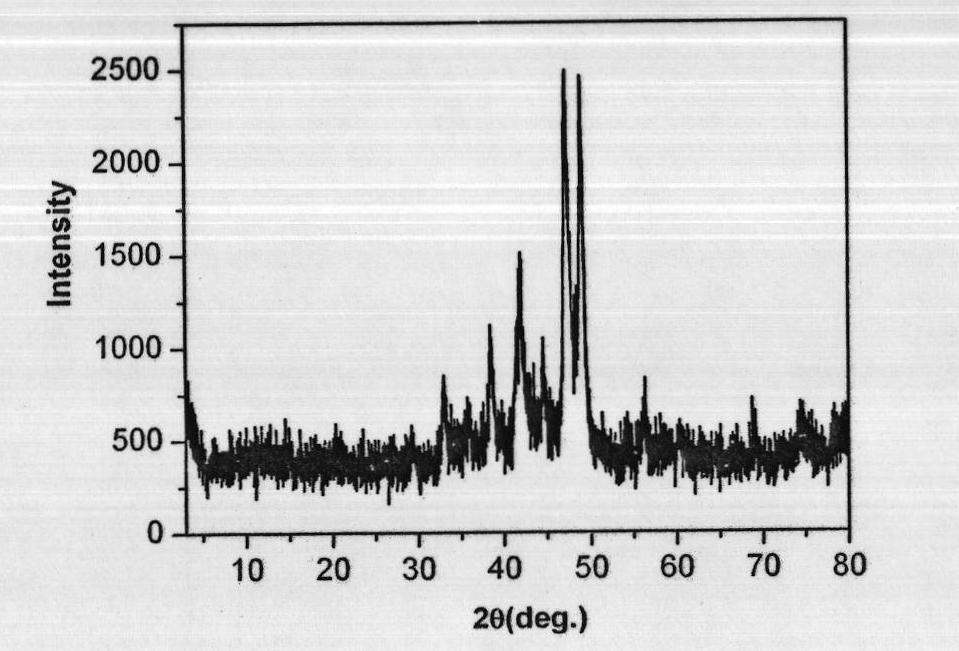
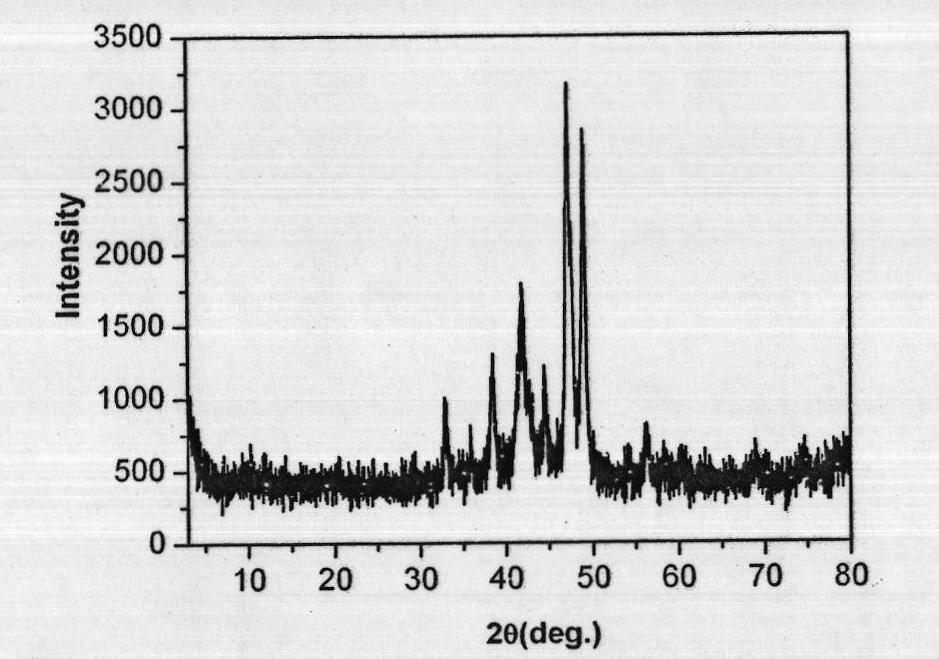
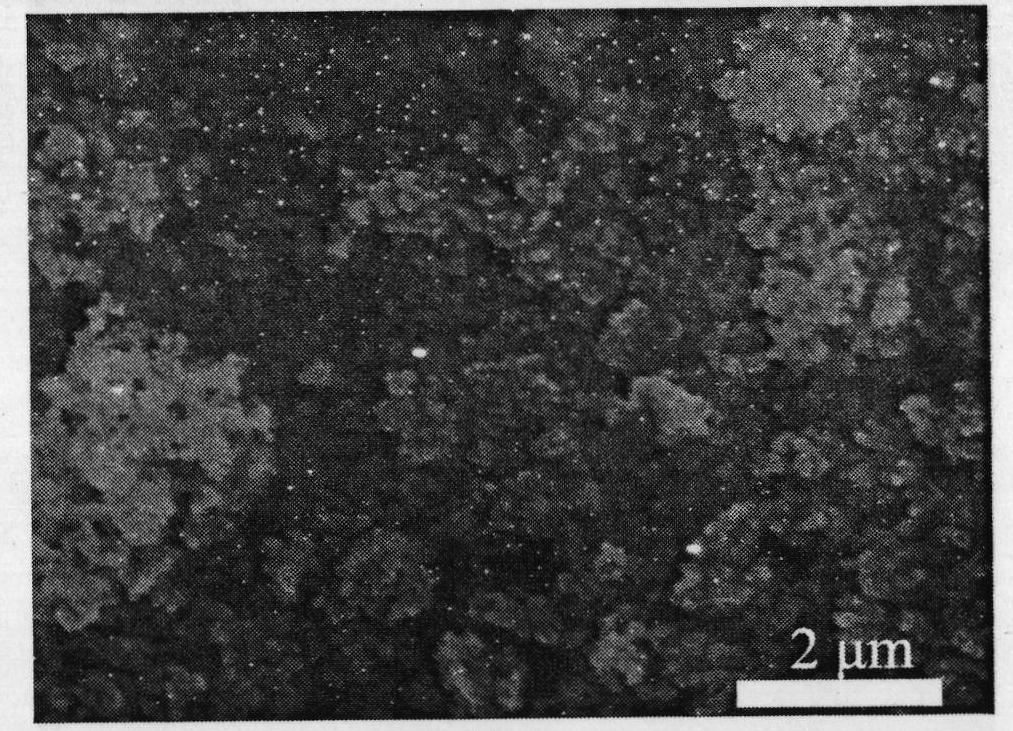
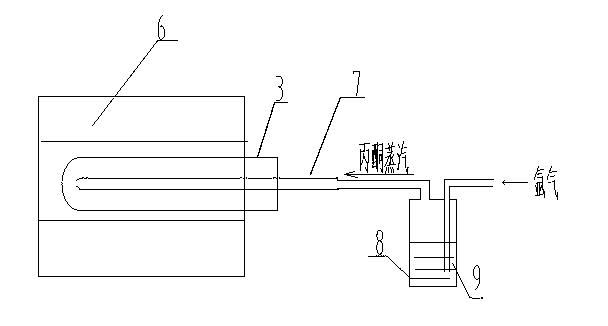
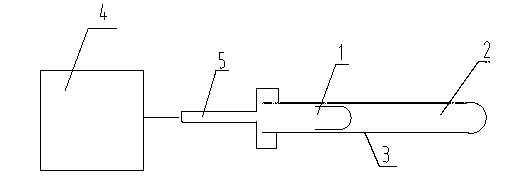
Preparation method of high-purity zinc phosphide
The invention discloses a preparation method of high-purity zinc phosphide, which comprises the following steps of: 1) quartz tube pretreatment; 2) dosing; 3) tube sealing; 4) high-temperature synthesis; and 5) product treatment. According to the invention, high-purity phosphorus and high-purity zinc are directly combined to produce high-purity zinc phosphide, the main reaction process is carried out after the temperature reaches the melting point of zinc, the reaction is actually a steam reaction, and the method is an element direct synthesis method; the requirements for the form of raw materials are not very strict; the raw material is not processed, and the requirement for high purity is met; the pressure in a tube is controlled through temperature control operations such as slow heating, constant temperature, slow cooling and the like; the phosphorus steam pressure in the quartz tube is successfully controlled through length allocation of a heating area and a condensation area for synthesizing a quartz tube, and safe synthesis of a large amount of zinc phosphide is realized; and relatively high yield of a product is realized by controlling the length of the condensation area and the heating area, and the product is applied to the application fields of photoelectric device manufacturing, diffusion sources, nano materials and the like.
Owner:峨嵋半导体材料研究所
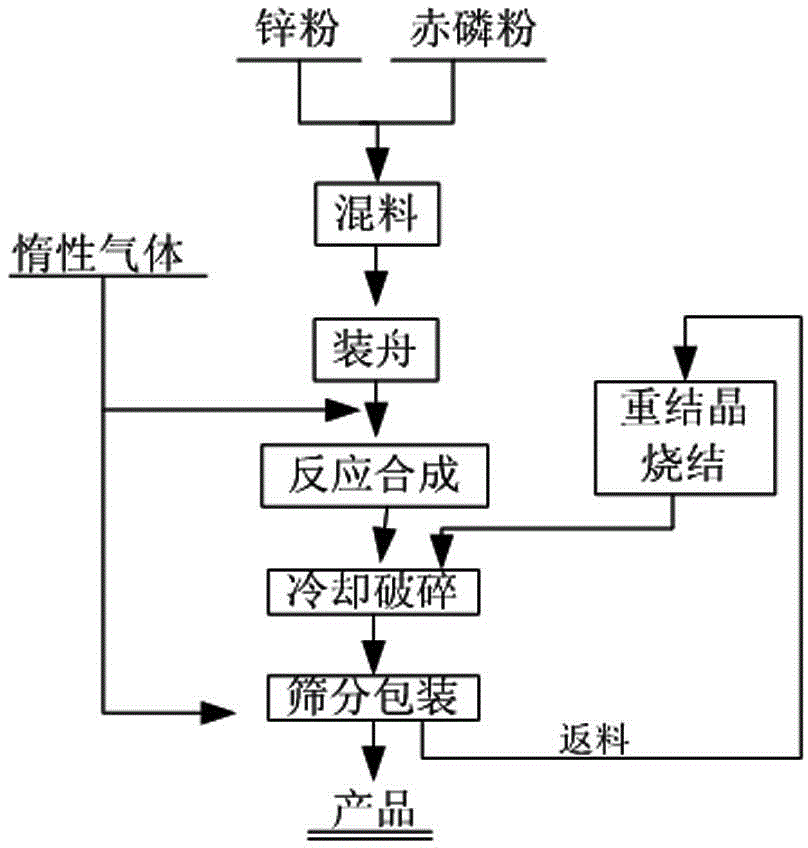
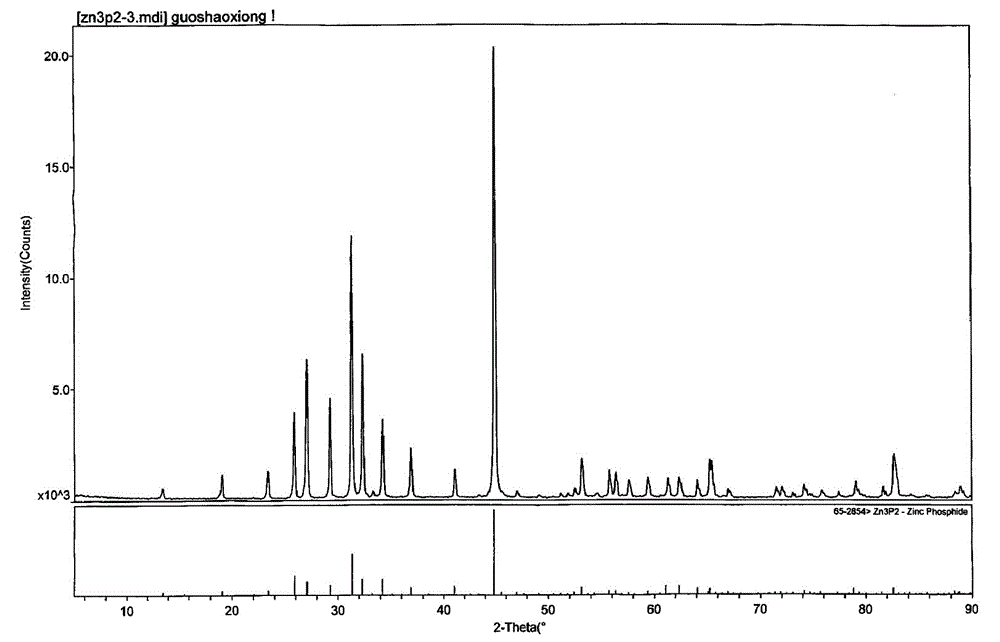
Preparation method of zinc phosphide
The invention relates to a preparation method of zinc phosphide. The invention belongs to a production method of chemical materials and in particular relates to a preparation method of tetragonal system high-purity zinc phosphide. The preparation method of zinc phosphide comprises the following steps: firstly, uniformly mixing metal zinc powder with red phosphorus powder in mass ratio of (3.1-3.3):1, placing mixture into a high-purity graphite boat, then placing the high-purity graphite boat into a smelting furnace while protective gas is introduced until pressure is 90-115KPa, and sealing the smelting furnace; secondly, slowly heating and warming at a speed of 0.5-35 DEG C / min, and carrying out synthetic reaction at the temperature of 100-900 DEG C, so that blocky zinc phosphide is obtained. The zinc phosphide obtained by adopting the preparation method is tetragonal system Zn3P2, product composition phase is uniform, purity is high, flow is short, a production technology is simple, and industrialization can be realized.
Owner:云南锡业研究院有限公司研究设计院
Preparation method for nickel phosphide-nitrogen doped graphite oxide foil composite nano material
InactiveCN108543543AEase of mass productionImprove flexibilityPhysical/chemical process catalystsElectrodesThioureaCatalytic effect
The invention discloses a preparation method for a nickel phosphide-nitrogen doped graphite oxide foil composite nano material. Graphite foil, HNO3, H2SO4, ammonium fluoride, thiourea, nickel nitrateand ultrapure water are used as raw materials; and the method is realized by the following steps: graphite oxide foil doped by nitrogen and sulfur atoms is prepared; a hydrothermal synthesis reactionis performed; and washing is performed, and drying is performed. The method disclosed by the invention adopts a hydrothermal method as a preparation process, the raw materials are simple and easy to obtain, the composite material is obtained through the hydrothermal and phosphating processes, the experiment process is simple, the operation is convenient, the large-scale production is easy to realize, and the product can be recovered by 100% during use; the obtained nickel phosphide-nitrogen doped graphite oxide foil has excellent flexibility, a nitrogen element is not only doped on the graphite foil, but also can enter crystal lattices of nickel phosphide, the composite nano material has a very good catalytic effect on electrocatalytic hydrogen evolution, and in the catalytic process, theproduct has good stability and good product uniformity; and in addition, the material is expected to have a good application in aspects of flexible batteries, flexible sensors and the like.
Owner:CHONGQING UNIV OF ARTS & SCI
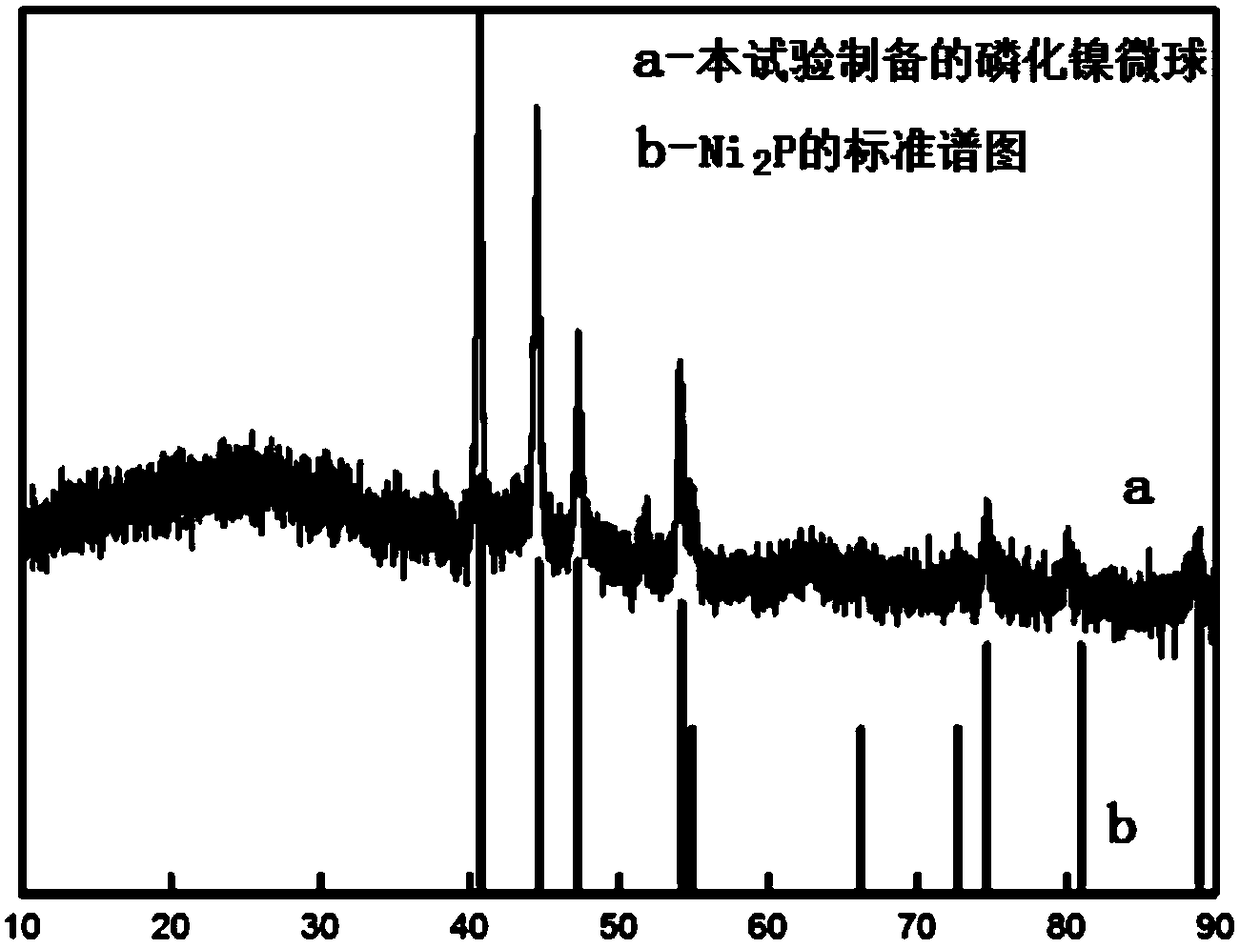
Preparation method of nickel phosphide microsphere with multilevel structure
InactiveCN108847486AHigh specific surface areaHigh tap densityMaterial nanotechnologyCell electrodesMicrosphereMetallic Nickel
The invention discloses a preparation method of a nickel phosphide microsphere with a multilevel structure, and relates to a preparation method of nickel phosphide. The method aims to solve the technical problem that the existing nickel phosphide is low in tap density. The method comprises the following steps of 1, preparing a precursor by using nickel acetate and urea; 2, putting the precursor into a porcelain boat, and then putting sodium hypophosphite into another porcelain boat, putting the two porcelain boats into a quartz tube together, and continuously pumping argon into the quartz tubefor protection, wherein the porcelain boat containing sodium hypophosphite is placed on the upstream of the air flow, and then heating the quartz tube to 300-400 DEG C, and carrying out heat preservation for 4-6 hours to obtain the nickel phosphide microsphere with the multilevel structure. The nickel phosphide microsphere disclosed by the invention is composed of a nickel phosphide nanosheet with a secondary structure, and the specific surface area of the nickel phosphide microsphere can be improved by utilizing the multilevel structure of the nickel phosphide microsphere, the contact area with an electrolyte is enlarged, the tap density is high, and the volume specific capacity is higher. The preparation method is simple and mild in conditions. The method can be used for manufacturing anegative electrode of a lithium ion battery.
Owner:HARBIN INST OF TECH
Point rail having hardened layer on working surface and its point rail working surface laser hardening treatment technology
InactiveCN1537956AImprove wear resistanceExtended service lifeRail switchesOptoelectronicsMachine tool
A point rail with the hardened layer on its working surface is disclosed. A technology for hardening its working surface by laser includes such steps as removing oily dirt and rust, baking, coating zinc phosphide paint for blackening it, and using CO2 laser device to harden it. Its advantages are high effect and productivity and easy control.
Owner:济南钢铁集团新事业有限公司
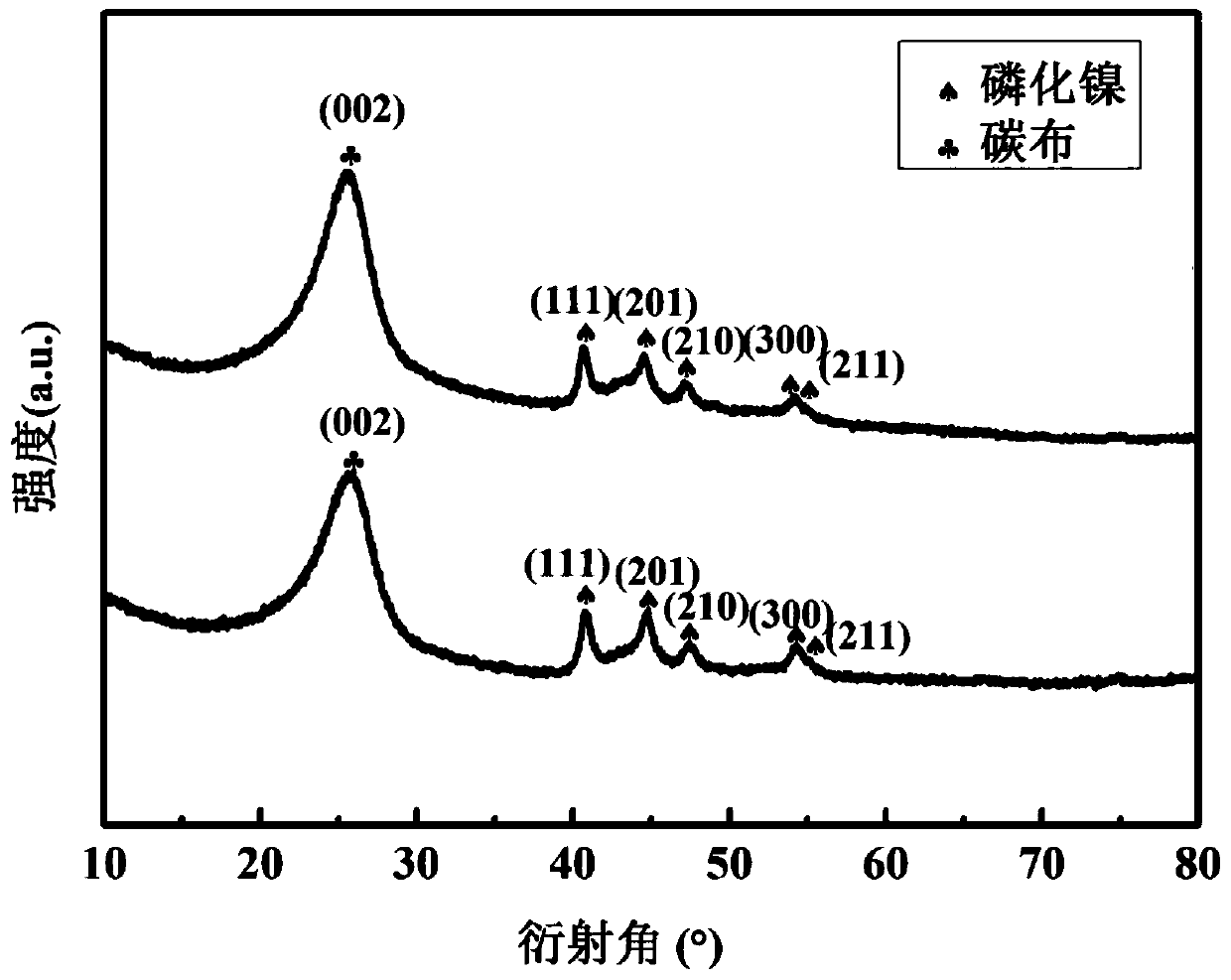
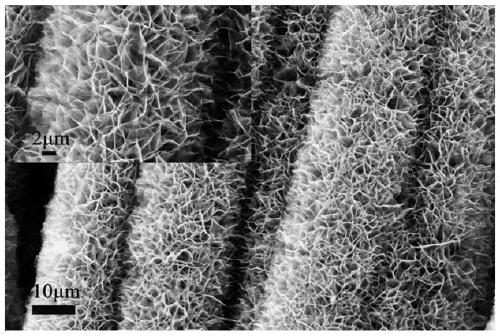
Preparation method of Ni-P/nickel phosphide-carbon cloth three-dimensional self-supporting hydrogen evolution electrode material
The invention relates to a preparation method of a Ni-P / nickel phosphide-carbon cloth three-dimensional self-supporting hydrogen evolution electrode material. The invention belongs to the technical field of hydrogen electrode material preparation. The preparation method comprises the following steps: performing hydrothermal reaction on carbon cloth subjected to acid impregnation modification treatment to grow nickel hydroxide; nickel hydroxide is subjected to phosphating treatment, a nickel phosphide-carbon cloth three-dimensional self-supporting material is obtained, then a nickel-phosphorusalloy is electrically deposited on nickel phosphide-carbon cloth, and then the nickel-phosphorus / nickel phosphide-carbon cloth three-dimensional self-supporting electrode material which does not needadditional bonding and is excellent in electro-catalytic hydrogen evolution performance is prepared. The preparation method has the advantages that the technical process is simple, the cost is low, industrial application is easy, the prepared electrode material is rich and intercommunicated in internal pore channels, the conductivity is excellent, the specific surface area is large, the number ofelectrocatalytic active sites is large, and the prepared nickel phosphorus / nickel phosphide-carbon cloth three-dimensional self-supporting electrode material has a good application prospect in the fields of hydrogen energy development and application.
Owner:YANSHAN UNIV
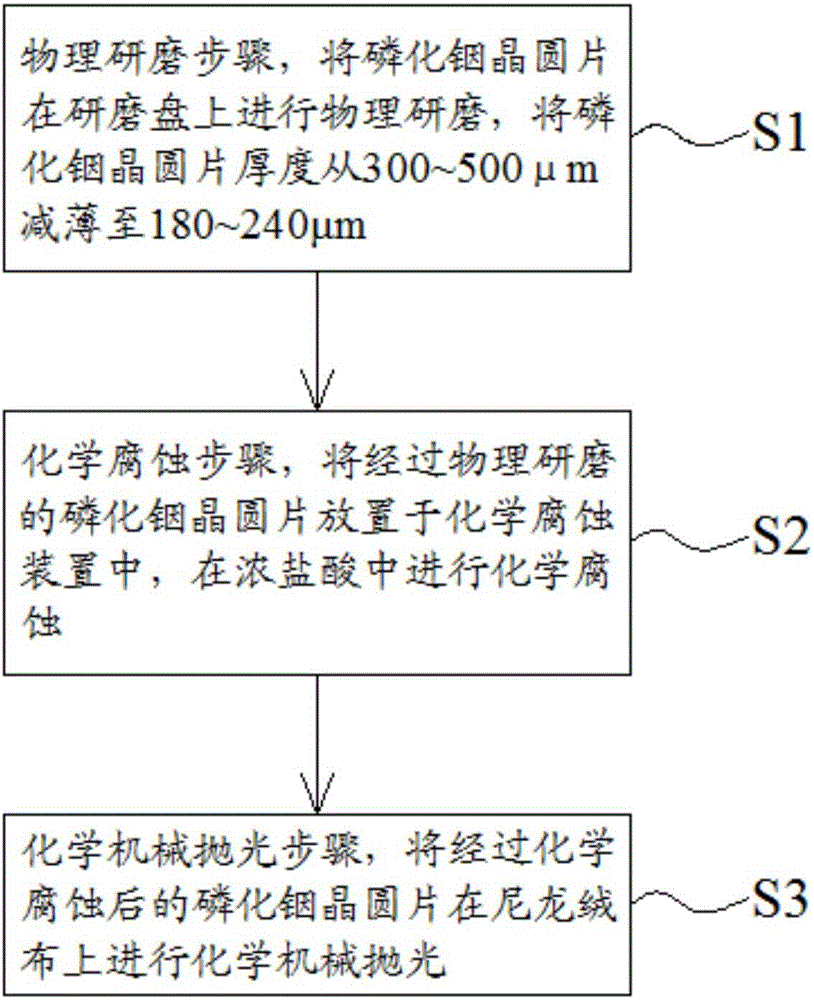
InP (indium phosphide) wafer thinning and polishing method and chemical corrosion device
InactiveCN106346318AOvercoming rateOvercome surface scratchesPolishing machinesLapping machinesWafer thinningChemical corrosion
Owner:SUZHOU EVERBRIGHT PHOTONICS CO LTD
Biological control method of longhorn beetles on trees
InactiveCN107155735AStrong targetingReduce pollutionBiocideDead animal preservationEcological environmentLonghorn beetle
The invention belongs to the technical field of longhorn beetle control and specifically discloses a biological control method of longhorn beetles on trees. The method comprises following steps: applying longhorn beetle larva control agent in the larva stage; applying longhorn beetle pupa control agent in the pupa stage; applying longhorn beetle imago control agent in the imago eclosion hole stage; in this way, the control of longhorn beetles on trees is completed; the longhorn beetle larva control agent is prepared from beauveria bassiana spore liquid, Sichuan azedarach extract, ethanol, and emulsifier; the longhorn beetle pupa control agent is prepared from beauveria bassiana spore liquid, adonis aestivalis extract and ethanol; the longhorn beetle imago control agent is prepared from beauveria bassiana spore liquid, hydrogen peroxide, ethanol and adonis aestivalis extract. According to the method of the invention, beauveria bassiana spore liquid is adopted as biological source for controlling longhorn beetles and the application of toxic chemical pesticides such as zinc phosphide and aluminum phosphide is avoided to reduce environment pollution and protect the ecological environment.
Owner:何振贤
Normal-temperature thick-film zinc phosphide phosphating solution and experimental method for determining component proportions
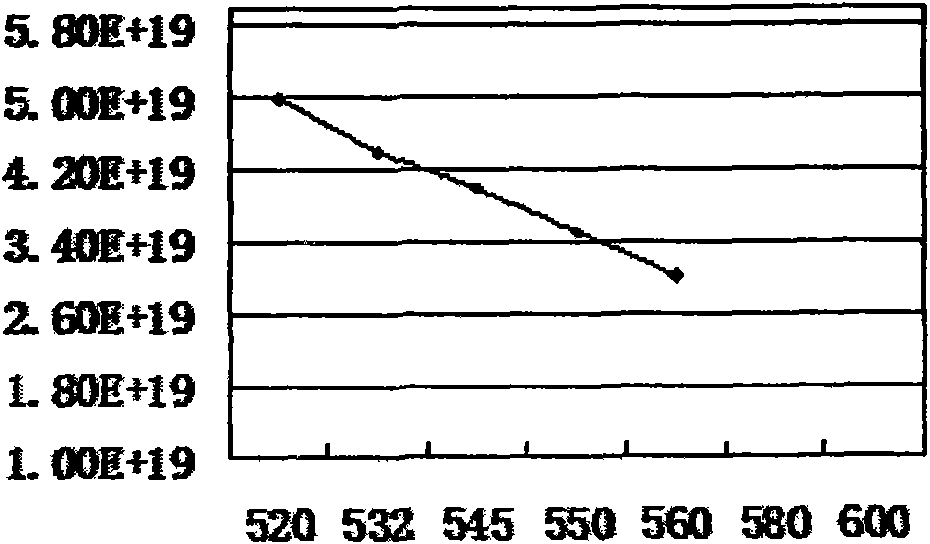
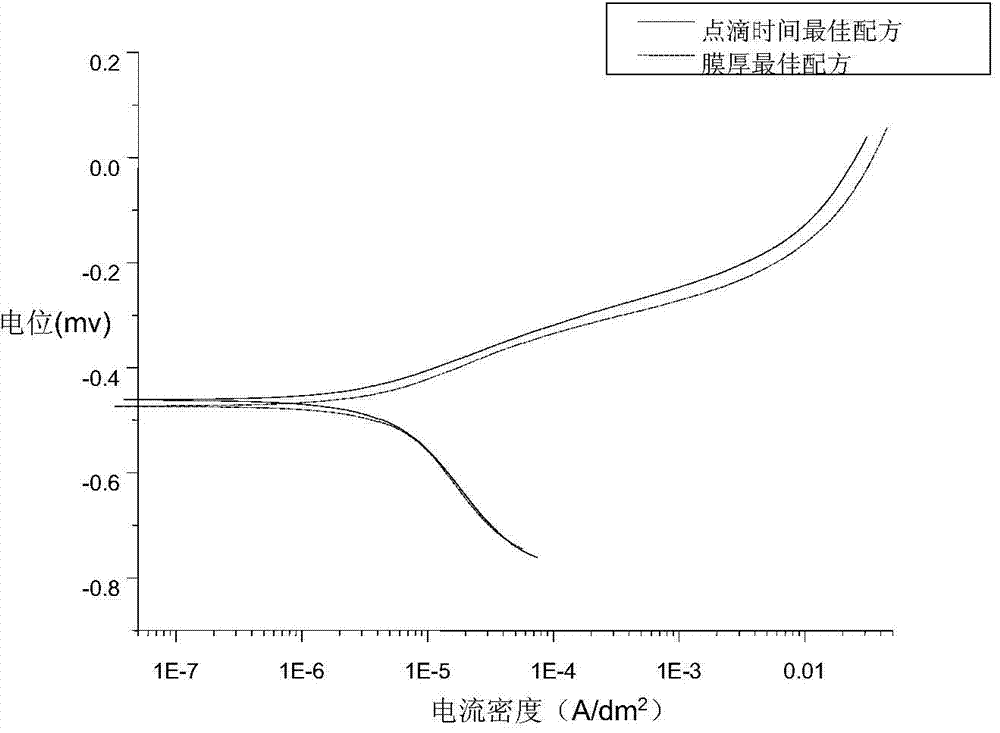
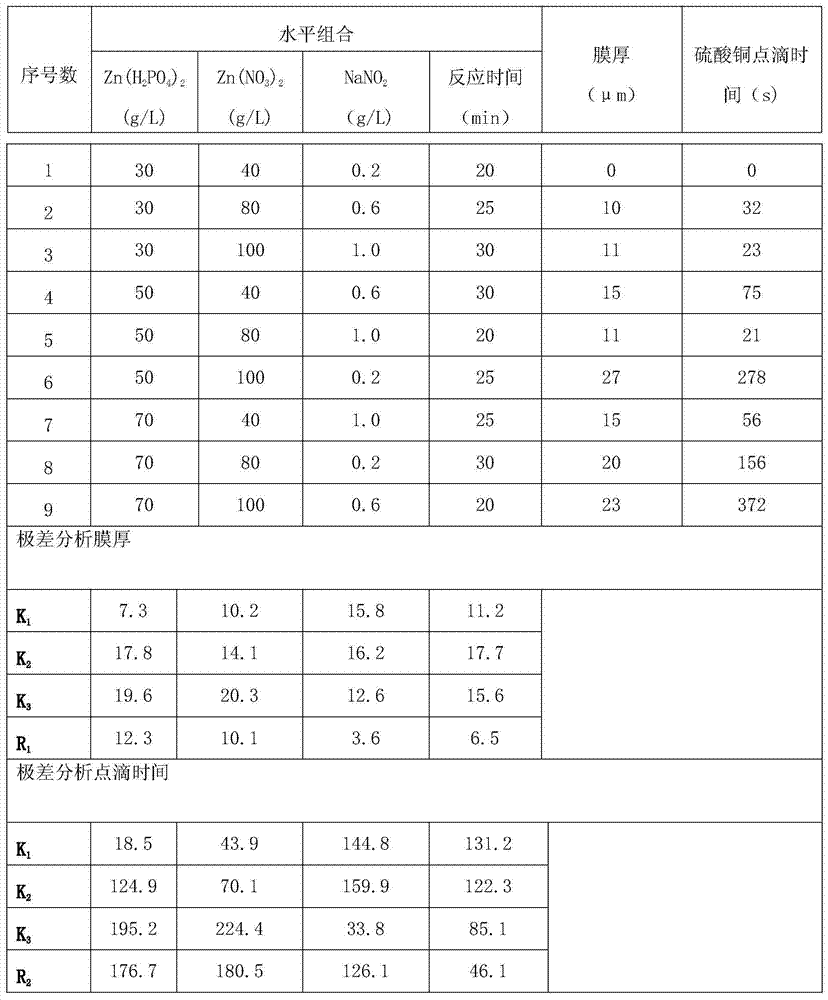
InactiveCN104264141AImprove corrosion resistanceCrystal denseMetallic material coating processesPorosityTest performance
The invention discloses a normal-temperature thick-film zinc phosphide phosphating solution and an experimental method for determining component proportions. The normal-temperature thick-film zinc phosphide phosphating solution comprises 270g / L of Zn(H2PO4), 100g / L of Zn(NO3)2, 0.6g / L of NaNO2, 6g / L of Ni(NO3)2, 10g / L of Ce(NO3)3.6(H2O), 1.5g / L of citric acid, and 1ml / L of an OP emulsifier, wherein the time is 20 minutes; the experimental method for determining component proportions of the normal-temperature thick-film zinc phosphide phosphating solution comprises the following steps: preparing a solution; preparing a phosphating film; and testing performances. Due to the normal-temperature thick-film zinc phosphide phosphating solution comprising the components, the formed phosphating film is dark grey and free of hanging ash; the film is even and continuous and has complete coverage; the relationship between film weight and film thickness is about 1 to 1; the average film weight exceeds 18.13g / L; the obtained phosphating film is a heavy film and is mainly used for preventing stored spare parts from being corroded; and the phosphating film is fine in crystals, small in porosity, good in corrosion resistance, and excellent in film corrosion resistance.
Owner:SOUTHWEST PETROLEUM UNIV
Cultivation method of southern magnolia
InactiveCN106105641AIncrease in the number of new shootsImprove stress resistancePlant cultivationCultivating equipmentsCarbofuranKerosene
The invention discloses a cultivation method of southern magnolia. Before storage, seeds are mixed into kerosene or processed by zinc phosphide in order to reduce damage of mouse insects to seeds. Seedbeds are disinfected before seedling growing such that survival rate after seedlings growing is increased. The composition and ratio of nutrition soil help thicken stems of southern magnolia, increase the number of fresh branches, reduce the number of dead branches and improve stress resistance of plants. Plant ashes and carbofuran are scattered on long matted beds so that underground pests are prevented and seedlings are lifted. The cultivation method is adopted so that southern magnolia has advantages of good stress resistance and robust growth. Therefore, a huge market potential and broad prospect are obtained.
Owner:ANHUI MEILAN LANDSCAPE ENG CO LTD
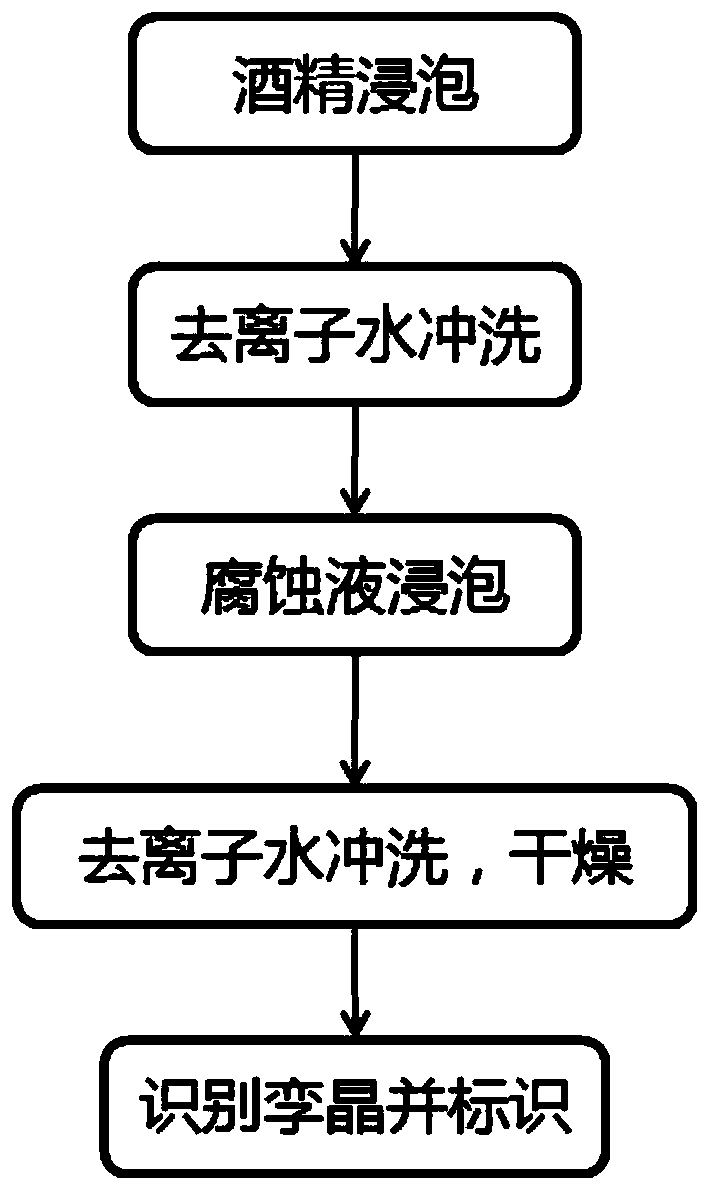
Corrosion liquid and corrosion method for identifying twin crystals on surface of indium phosphide
ActiveCN110453289AEasy to identifyImprove stabilityPolycrystalline material growthAfter-treatment detailsSemiconductor materialsAluminum phosphide
Owner:广东先导微电子科技有限公司
Preparation method of nickel phosphide catalyst carried by composite carrier
InactiveCN101612584BLittle loss of activityHigh activityCatalyst activation/preparationRefining to eliminate hetero atomsNickel saltPhosphate
The invention relates to a preparation method of a nickel phosphide catalyst carried by a composite carrier. The method is characterized by comprising the following steps: firstly, using activated alumina and relative enzymes and alcohols as raw materials to prepare a composite carrier of TiO2-Al2O3 by adopting an improved sol-gel method; further, selecting suitable nickel salt and phosphate and carrying out ultrasound dipping and drying in microwaves to obtain a catalyst precursor; and finally, increasing a temperature by a program and reducing to obtain the composite support. The composite carrier of TiO2-Al2O3 prepared by the invention not only can effectively overcome the defect that nickel phosphide is apt to react with gamma-Al2O3 to generate AlP4 to lose the activity, but also overcomes the defect that a specific surface area of single TiO2 is relatively small, effectively exerts the strong metal-support interaction (SMSI) of the TiO2, thereby obtaining a hydrodesulfurization catalyst with remarkable activity efficacy.
Owner:XI AN JIAOTONG UNIV
Preparation method of InP/ZnS (indium phosphide/zinc sulfide) core-shell structure quantum dots
InactiveCN107502352AUniform particle size distributionHigh fluorescence yieldNanoopticsLuminescent compositionsIndiumIodide
The invention discloses a preparation method of InP / ZnS (indium phosphide / zinc sulfide) core-shell structure quantum dots. The preparation method comprises a preparation method of InP-series core-shell quantum dots and a synthesis method of the InP / ZnS core-shell structure quantum dots; the preparation method of InP-series core-shell quantum dots is used for preparation of a phosphorus source; the synthesis method is used for preparing an indium precursor with indium iodide as a quantum dot, and the InP / ZnS core-shell structure quantum dots are obtained with P4 as the phosphorus source, dodecanethiol as an S source, 1-octodecene as a stabilizer, oleylamine as a solvent reactant and a ligand as well as zinc oleate as a zinc source of a coated shell structure; centrifugal separation and purification are performed after the reaction is performed; pure InP / ZnS core-shell structure quantum dots are dispersed in octane again; the purified quantum dots are assembled into a QLED (quantum dot light-emitting diode) light emitting device. The preparation method has the advantages as follows: the yield of organic phase synthesized quantum dots is high, the fluorescent yield of a film is high, the quantum dots have uniformly distributed sizes, luminescent spectra are symmetric and narrow, and the cost is greatly reduced by use of the cheap phosphorus source.
Owner:NANCHANG HANGKONG UNIVERSITY
Preparation method of highly dispersed supported nickel phosphide catalyst
ActiveCN110215927AGood HDO activityGood dispersionMolecular sieve catalystsLiquid hydrocarbon mixture productionDispersitySodium acetate
The invention provides a preparation method of a supported nickel phosphide catalyst for catalyzing hydrodeoxygenation of phenol and a derivative thereof. The catalyst is supported nickel phosphide. The preparation method comprises the following steps: 1, taking nickel nitrate hexahydrate and dissolving nickel nitrate hexahydrate in deionized water to form a solution; 2, adding a carrier, and performing continuous stirring and heating; 3, weighing urea and adding urea into the obtained solution, and adding concentrated nitric acid; 4, dropwise adding the solution obtained in the step 3 into the suspension solution in the step 2, performing heating after the drop addition is completed, and continuously carrying out a reaction; 5, performing vacuum filtration, performing washing with deionized water until a filtrate is neutral, and performing drying in an oven overnight to obtain a gray-black solid; 6, preparing an acetic acid-sodium acetate buffer solution, then adding sodium hypophosphite and performing continuous stirring and heating, and slowly adding a precursor compound; and 7, performing vacuum filtration after a reaction, performing washing with deionized water until a filtrate is neutral, performing drying in an oven overnight, performing heat treatment in a chemical atmosphere, and then performing cooling annealing to obtain the supported nickel phosphide catalyst. Thecatalyst obtained by the method has good dispersity, small particle size and good HDO activity.
Owner:DALIAN UNIV OF TECH
Preparation of nano-flaky iron-doped nickel phosphide and nitrogen reduction reaction (NRR) application
InactiveCN109876835AImprove efficiencyGood effectMaterial nanotechnologyCatalyst activation/preparationElectrolysisFaraday efficiency
In view of the heavy demand for ammonia and the harsh reaction condition and low conversion rate of a Haber-Bosch process, simple preparation of the ammonia becomes a major problem of development of the present world, and therefore, the study of achieving nitrogen reduction ammonia through electrolysis of an electrolyte with saturated nitrogen at the normal temperature and normal pressure is paidmuch attention. The invention provides a preparation method of nano-flaky iron-doped nickel phosphide nano-powder and application of the nano-flaky iron-doped nickel phosphide nano-powder to a nitrogen reduction reaction (NRR). The preparation method comprises the steps: firstly, a specific proportion of iron source reagent and nickel source reagent are added into a reaction solution, a heating reaction is conducted, and iron-nickel precursor nano-powder is obtained; and then the iron-nickel precursor nano-powder is placed into a tube furnace at the specific nitrogen flow rate to be subjectedto a phosphorization reaction, and finally the nano-flaky iron-doped nickel phosphide nano-powder is obtained. The nano-flaky iron-doped nickel phosphide nano-powder shows excellent catalytic activityin the field of the NRR, the ammonia yield under minus 0.3 V (relative to a standard hydrogen electrode) reaches up to 70.6 [mu]g h<-1> mg<cat.><-1>, and the Faradic efficiency reaches 6.5%.
Owner:UNIV OF JINAN
Blue light cadmium-free quantum dot, preparation method thereof and quantum dot photoelectric device
ActiveCN111849483AOptimizing the band structureSmall sizeMaterial nanotechnologySolid-state devicesIndiumCarboxylic acid
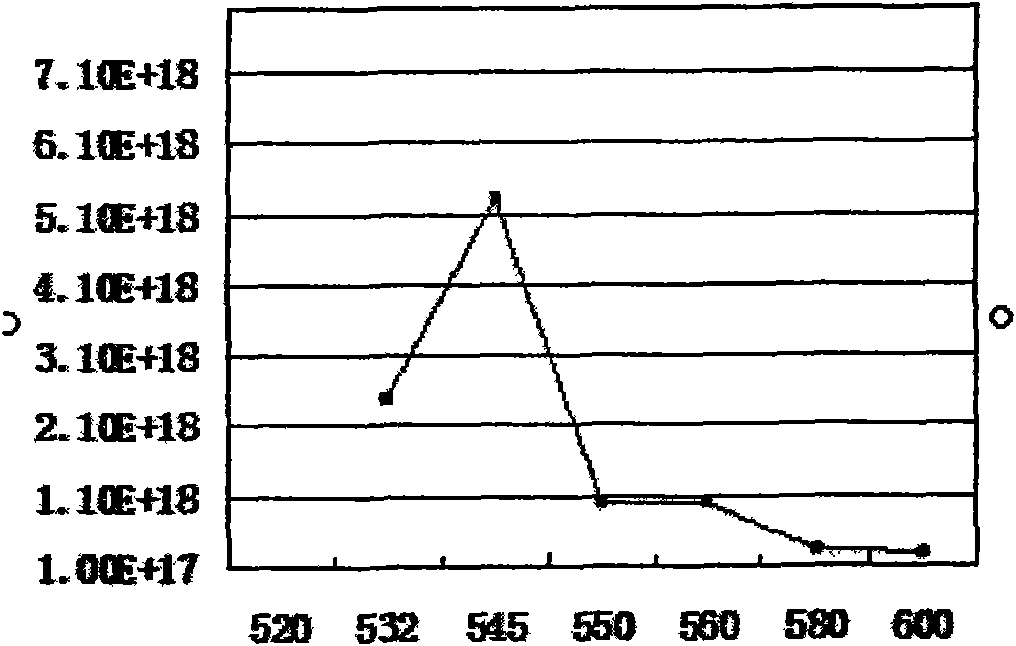
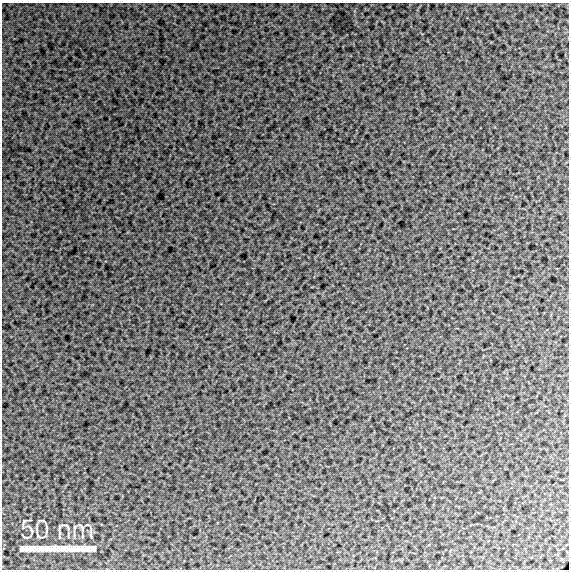
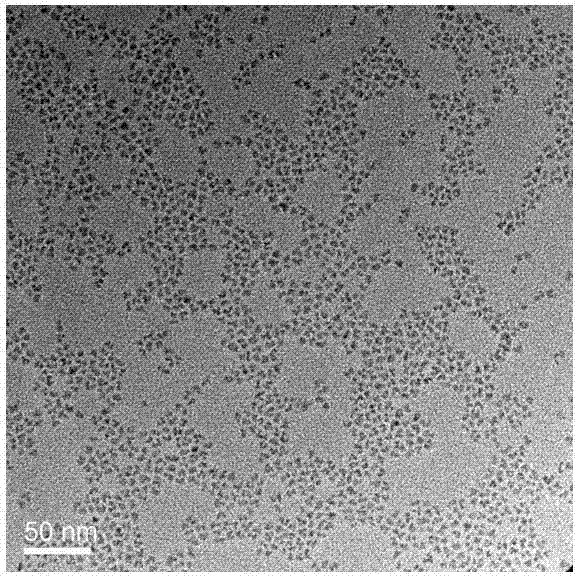
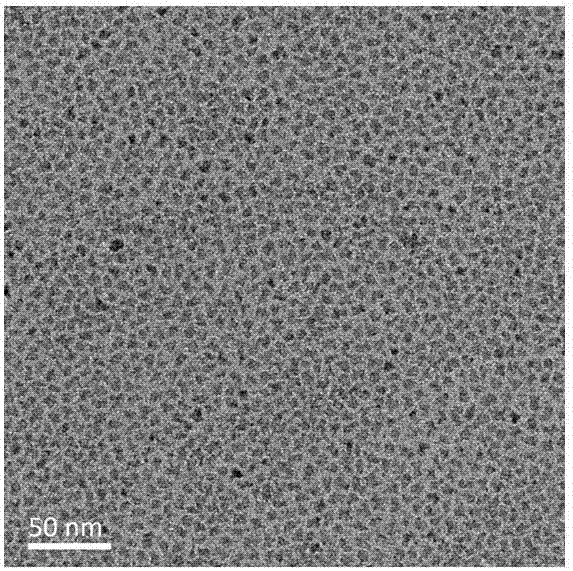
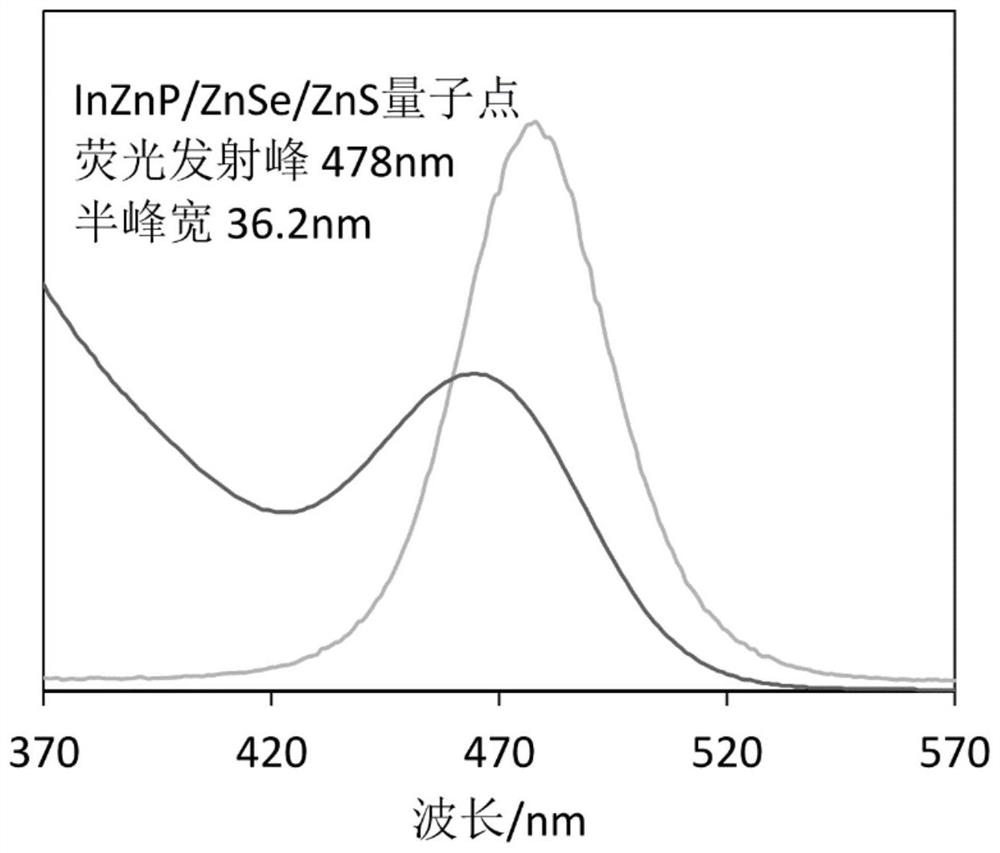
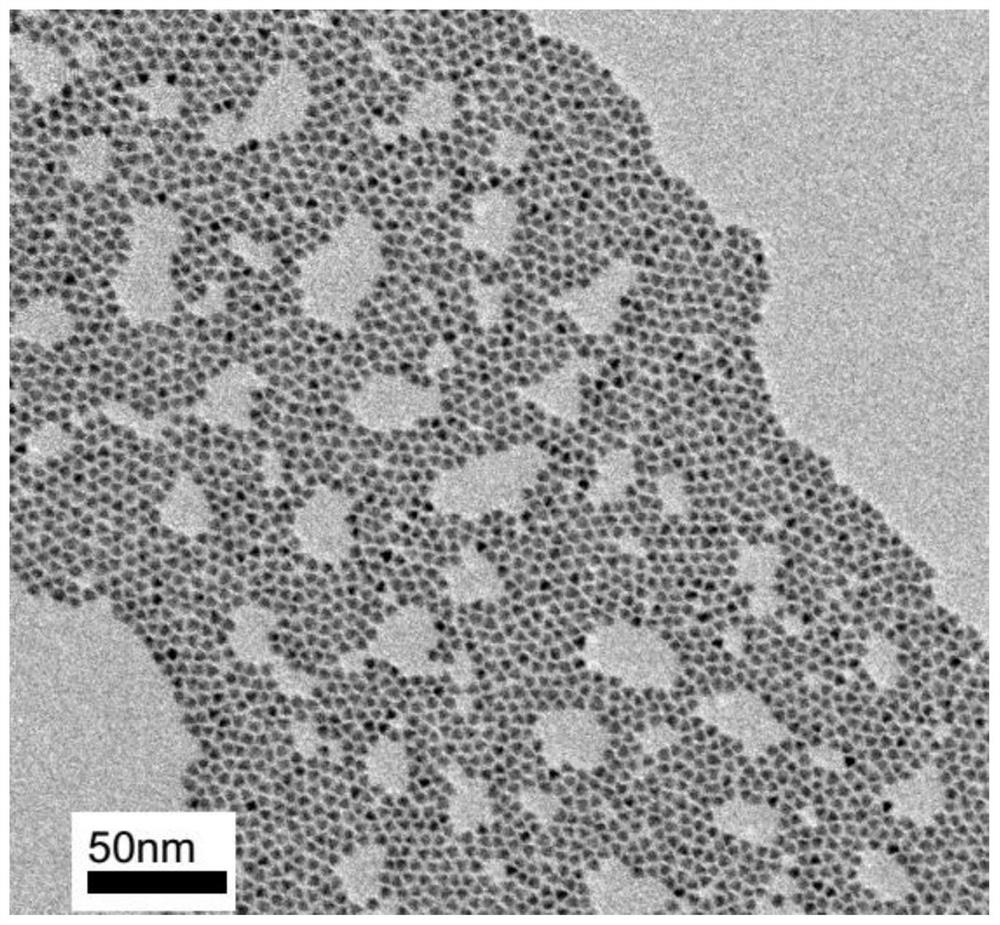
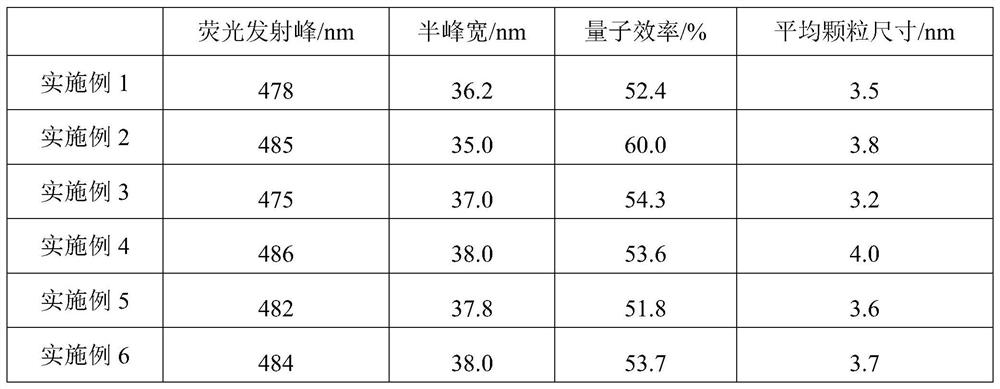
The invention discloses a blue light cadmium-free quantum dot, a preparation method thereof and a quantum dot light-emitting device. The preparation method of the cadmium-free quantum dot comprises the following steps that S1, an indium precursor, a first ligand and a solvent are mixed to prepare an indium-first ligand solution, the first ligand is a long-chain carboxylic acid ligand with the carbon number being 10-18, and the ratio of the amount of substance of carboxylate radicals of the first ligand to the amount of substance of indium ions of the indium precursor is (1: 2)-(5: 2); S2, short-chain zinc salt and a second ligand are added into the indium-first ligand solution to form a cation-mixed ligand solution, and at least part of the short-chain zinc salt and the second ligand are bonded through coordination bonds; S3, a phosphorus precursor is added into the cation-mixed ligand solution to react to obtain a solution containing indium zinc phosphide quantum dots. The InZnP quantum dot core with small size and few defects is prepared by introducing the short-chain zinc salt in the nucleation process of the quantum dot, and the introduction of the Zn element also optimizes theenergy band structure of the quantum dot so that the quantum dot with the luminescence range of 470-490nm is obtained.
Owner:NANJING TECH CORP LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com